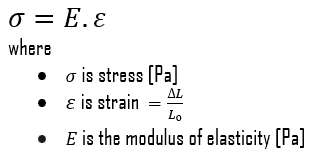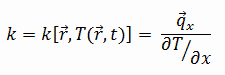Brass
 Brass is is the generic term for a range of copper-zinc alloys. Brass can be alloyed with zinc in different proportions, which results in a material of varying mechanical, corrosion and thermal properties. Increased amounts of zinc provide the material with improved strength and ductility. Brasses with a copper content greater than 63% are the most ductile of any copper alloy and are shaped by complex cold forming operations. Brass has higher malleability than bronze or zinc. The relatively low melting point of brass and its fluidity make it a relatively easy material to cast. Brass can range in surface color from red to yellow to gold to silver depending on the zinc content. Some of the common uses for brass alloys include costume jewelry, locks, hinges, gears, bearings, hose couplings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins. Brass and bronze are common engineering materials in modern architecture and primarily used for roofing and facade cladding due to their visual appearance.
Brass is is the generic term for a range of copper-zinc alloys. Brass can be alloyed with zinc in different proportions, which results in a material of varying mechanical, corrosion and thermal properties. Increased amounts of zinc provide the material with improved strength and ductility. Brasses with a copper content greater than 63% are the most ductile of any copper alloy and are shaped by complex cold forming operations. Brass has higher malleability than bronze or zinc. The relatively low melting point of brass and its fluidity make it a relatively easy material to cast. Brass can range in surface color from red to yellow to gold to silver depending on the zinc content. Some of the common uses for brass alloys include costume jewelry, locks, hinges, gears, bearings, hose couplings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins. Brass and bronze are common engineering materials in modern architecture and primarily used for roofing and facade cladding due to their visual appearance.
 For example, UNS C26000 cartridge brass alloy (70/30) is from the yellow brass series, which has the highest ductility. Cartridge brasses are mostly cold formed and they can also be easily machined, which is necessary in making cartridge cases. It can be used for radiator cores and tanks, flashlight shells, lamp fixtures, fasteners, locks, hinges, ammunition components or plumbing accessories.
For example, UNS C26000 cartridge brass alloy (70/30) is from the yellow brass series, which has the highest ductility. Cartridge brasses are mostly cold formed and they can also be easily machined, which is necessary in making cartridge cases. It can be used for radiator cores and tanks, flashlight shells, lamp fixtures, fasteners, locks, hinges, ammunition components or plumbing accessories.
Steels
Steels are iron–carbon alloys that may contain appreciable concentrations of other alloying elements. Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater ductility. Due to its very-high strength, but still substantial toughness, and its ability to be greatly altered by heat treatment, steel is one of the most useful and common ferrous alloy in modern use. There are thousands of alloys that have different compositions and/or heat treatments. The mechanical properties are sensitive to the content of carbon, which is normally less than 1.0 wt%. According ot AISI classification, carbon steel is broken down into four classes based on carbon content.
Types of Steels – Classification Based on Composition
-

Typical applications for low-carbon steel include automobile body components, structural shapes (e.g., I-beams, channel and angle iron), and sheets that are used in pipelines, buildings. Steel. Steels are iron–carbon alloys that may contain appreciable concentrations of other alloying elements. Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater strength. Due to its very-high strength, but still substantial toughness, and its ability to be greatly altered by heat treatment, steel is one of the most useful and common ferrous alloy in modern use. There are thousands of alloys that have different compositions and/or heat treatments. The mechanical properties are sensitive to the content of carbon, which is normally less than 1.0 wt%. According ot AISI classification, carbon steel is broken down into four classes based on carbon content:
- Low-carbon Steels. Low-carbon steel, also known as mild steel is now the most common form of steel because its price is relatively low while it provides material properties that are acceptable for many applications. Low-carbon steel contains approximately 0.05–0.25% carbon making it malleable and ductile. Mild steel has a relatively low tensile strength, but it is cheap and easy to form; surface hardness can be increased through carburizing.
- Medium-carbon Steels. Medium-carbon steel has approximately 0.3–0.6% carbon content. Balances ductility and strength and has good wear resistance. This grade of steel is mostly used in the production of machine components, shafts, axles, gears, crankshafts, coupling and forgings and could also be used in rails and railway wheels.
- High-carbon Steels. High-carbon steel has approximately 0.60 to 1.00% carbon content. Hardness is higher than the other grades but ductility decreases. High carbon steels could be used for springs, rope wires, hammers, screwdrivers, and wrenches.
- Ultra-high-carbon Steels. Ultra-high-carbon steel has approximately 1.25–2.0% carbon content. Steels that can be tempered to great hardness. This grade of steel could be used for hard steel products, such as truck springs, metal cutting tools and other special purposes like (non-industrial-purpose) knives, axles or punches. Most steels with more than 2.5% carbon content are made using powder metallurgy.
- Alloy Steels. Steel is an alloy of iron and carbon, but the term alloy steel usually only refers to steels that contain other elements— like vanadium, molybdenum, or cobalt—in amounts sufficient to alter the properties of the base steel. In general, alloy steel is steel that is alloyed with a variety of elements in total amounts between 1.0% and 50% by weight to improve its mechanical properties. Alloy steels are broken down into two groups:
- Low-alloy Steels.
- High-alloy Steels.
- Stainless Steel. Stainless steels are defined as low-carbon steels with at least 10% chromium with or without other alloying elements. Strength and corrosion resistance often make it the material of choice in transportation and processing equipment, engine parts, and firearms. Chromium increases hardness, strength, and corrosion resistance. Nickel gives similar benefits but adds hardness without sacrificing ductility and toughness. It also reduces thermal expansion for better dimensional stability.
Stainless Steels
 In metallurgy, stainless steel is a steel alloy with at least 10.5% chromium with or without other alloying elements and a maximum of 1.2% carbon by mass. Stainless steels, also known as inox steels or inox from French inoxydable (inoxidizable), are steel alloys, which are very well known for their corrosion resistance, which increases with increasing chromium content. Corrosion resistance may also be enhanced by nickel and molybdenum additions. The resistance of these metallic alloys to the chemical effects of corrosive agents is based on passivation. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 10.5% by weight, above which passivity can occur and below which it is impossible. Chromium can be used as a hardening element and is frequently used with a toughening element such as nickel to produce superior mechanical properties.
In metallurgy, stainless steel is a steel alloy with at least 10.5% chromium with or without other alloying elements and a maximum of 1.2% carbon by mass. Stainless steels, also known as inox steels or inox from French inoxydable (inoxidizable), are steel alloys, which are very well known for their corrosion resistance, which increases with increasing chromium content. Corrosion resistance may also be enhanced by nickel and molybdenum additions. The resistance of these metallic alloys to the chemical effects of corrosive agents is based on passivation. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 10.5% by weight, above which passivity can occur and below which it is impossible. Chromium can be used as a hardening element and is frequently used with a toughening element such as nickel to produce superior mechanical properties.
Uses of Stainless Steels – Applications
Strength and corrosion resistance of stainless steel often make it the material of choice in transportation and processing equipment, engine parts, and firearms. Most of the structural applications occur in the chemical and power engineering industries, which account for more than third of the market for stainless steel products. The wide variety of applications includes nuclear reactor vessels, heat exchangers. The body of the reactor vessel is constructed of a high-quality low-alloy carbon steel, but all surfaces that come into contact with reactor coolant (highly corrosive due to the presence of boric acid) are clad with a minimum of about 3 to 10 mm of austenitic stainless steel in order to minimize corrosion.
Stainless steel can be rolled into sheets, plates, bars, wire, and tubing. Stainless steels do not need to be painted or coated, which makes them suitable for use in applications where cleanliness is required: in cookware, cutlery and surgical instruments.
Types of Stainless Steels
Stainless steel is a generic term for a large family of corrosion resistant alloys containing at least 10.5% chromium and may contain other alloying elements. There are numerous grades of stainless steel with varying chromium and molybdenum contents and with varying crystallographic structure to suit the environment the alloy must endure. Stainless steels can be divided into five categories:
- Ferritic stainless steels. In ferritic stainless steels, carbon is kept to low levels (C<0.08%) and the chromium content can range from 10.50 to 30.00%. They are usually limited in use to relatively thin sections due to lack of toughness in welds. Moreover, they have relatively poor high-temperature strength. Ferritic steels are chosen for their resistance to stress corrosion cracking, which makes them an attractive alternative to austenitic stainless steels in applications where chloride-induced SCC is prevalent.
- Austenitic stainless steels. Austenitic stainless steels contain between 16 and 25% Cr and can also contain nitrogen in solution, both of which contribute to their relatively high corrosion resistance. Austenitic stainless steels have the best corrosion resistance of all stainless steels and they have excellent cryogenic properties, and good high-temperature strength. The best known grade is AISI 304 stainless, which contains both chromium (between 15% and 20%) and nickel (between 2% and 10.5%) metals as the main non-iron constituents. 304 stainless steel has excellent resistance to a wide range of atmospheric environments and many corrosive media. These alloys are usually characterized as ductile, weldable, and hardenable by cold forming.
- Martensitic stainless steels. Martensitic stainless steels are similar to ferritic steels in being based on chromium but have higher carbon levels up as high as 1%. They are sometimes classified as low-carbon and high-carbon martensitic stainless steels. They have moderate corrosion resistance, but are considered hard, strong, slightly brittle. They are magnetic and they can be nondestructively tested using the magnetic particle inspection method, unlike austenitic stainless steel. A common martensitic stainless is AISI 440C, which contains 16 to 18% chromium and 0.95 to 1.2% carbon. Grade 440C stainless steel is used in the following applications: gage blocks, cutlery, ball bearings and races, molds and dies, knives.
- Duplex Stainless Steels. Duplex stainless steels, as their name indicates, are a combination of two of the main alloy types. They have a mixed microstructure of austenite and ferrite, the aim usually being to produce a 50/50 mix, although in commercial alloys the ratio may be 40/60. Their corrosion resistance is similar to their austenitic counterparts, but their stress-corrosion resistance (especially to chloride stress corrosion cracking), tensile strength, and yield strengths (roughly twice the yield strength of austenitic stainless steels) are generally superior to that of the austenitic grades. Superduplex steels have enhanced strength and resistance to all forms of corrosion compared to standard austenitic steels. Common uses are in marine applications, petrochemical plant, desalination plant, heat exchangers and papermaking industry. Today, the oil and gas industry is the largest user and has pushed for more corrosion resistant grades, leading to the development of superduplex steels.
- PH Stainless Steels. PH stainless steels (precipitation-hardening) contain around 17% chromium and 4% nickel. These steels can develop very high strength through additions of aluminum, titanium, niobium, vanadium, and/or nitrogen, which form coherent intermetallic precipitates during a heat treatment process referred to as heat aging. Of all of the available stainless grades, they generally offer the greatest combination of high strength coupled with excellent toughness and corrosion resistance. They are as corrosion resistant as austenitic grades. Common uses are in the aerospace and some other high-technology industries.
Properties of Brass vs Steel and Stainless Steel
Material properties are intensive properties, that means they are independent of the amount of mass and may vary from place to place within the system at any moment. The basis of materials science involves studying the structure of materials, and relating them to their properties (mechanical, electrical etc.). Once a materials scientist knows about this structure-property correlation, they can then go on to study the relative performance of a material in a given application. The major determinants of the structure of a material and thus of its properties are its constituent chemical elements and the way in which it has been processed into its final form.
Density of Brass vs Steel and Stainless Steel
Density of typical brass – UNS C26000 is 8.53 g/cm3.
Density of typical stainless steel is 8.0 g/cm3 (304 steel).
Density of typical steel is 8.05 g/cm3.
Density is defined as the mass per unit volume. It is an intensive property, which is mathematically defined as mass divided by volume:
ρ = m/V
In words, the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance. The standard SI unit is kilograms per cubic meter (kg/m3). The Standard English unit is pounds mass per cubic foot (lbm/ft3).
Since the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance, it is obvious, the density of a substance strongly depends on its atomic mass and also on the atomic number density (N; atoms/cm3),
- Atomic Weight. The atomic mass is carried by the atomic nucleus, which occupies only about 10-12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Therefore it is determined by the mass number (number of protons and neutrons).
- Atomic Number Density. The atomic number density (N; atoms/cm3), which is associated with atomic radii, is the number of atoms of a given type per unit volume (V; cm3) of the material. The atomic number density (N; atoms/cm3) of a pure material having atomic or molecular weight (M; grams/mol) and the material density (⍴; gram/cm3) is easily computed from the following equation using Avogadro’s number (NA = 6.022×1023 atoms or molecules per mole):
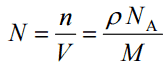
- Crystal Structure. Density of crystalline substance is significantly affected by its crystal structure. FCC structure, along with its hexagonal relative (hcp), has the most efficient packing factor (74%). Metals containing FCC structures include austenite, aluminum, copper, lead, silver, gold, nickel, platinum, and thorium.
Mechanical Properties of Brass vs Steel and Stainless Steel
Materials are frequently chosen for various applications because they have desirable combinations of mechanical characteristics. For structural applications, material properties are crucial and engineers must take them into account.
Strength of Brass vs Steel and Stainless Steel
In mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. Strength of materials basically considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. Strength of a material is its ability to withstand this applied load without failure or plastic deformation.
Ultimate Tensile Strength
Ultimate tensile strength of cartridge brass – UNS C26000 is about 315 MPa.
Ultimate tensile strength of stainless steel – type 304L is 485 MPa.
Ultimate tensile strength of low-carbon steel is between 400 – 550 MPa.
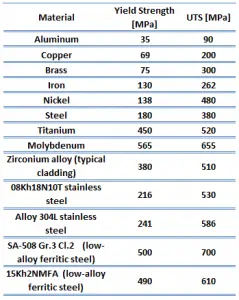 The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after the ultimate strength has been achieved. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for an aluminum to as high as 3000 MPa for very high-strength steels.
The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after the ultimate strength has been achieved. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for an aluminum to as high as 3000 MPa for very high-strength steels.
Yield Strength
Yield strength of cartridge brass – UNS C26000 is about 95 MPa.
Yield strength of stainless steel – type 304L is 170 MPa.
Yield strength of low-carbon steel is 250 MPa.
The yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning plastic behavior. Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically whereas yield point is the point where nonlinear (elastic + plastic) deformation begins. Prior to the yield point, the material will deform elastically and will return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible. Some steels and other materials exhibit a behaviour termed a yield point phenomenon. Yield strengths vary from 35 MPa for a low-strength aluminum to greater than 1400 MPa for very high-strength steels.
Young’s Modulus of Elasticity
Young’s modulus of elasticity of cartridge brass – UNS C26000 is about 110 GPa.
Young’s modulus of elasticity stainless steel – type 304 and 304L is 193 GPa.
Young’s modulus of elasticity of low-carbon steel is 200 GPa.
The Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests. Up to a limiting stress, a body will be able to recover its dimensions on removal of the load. The applied stresses cause the atoms in a crystal to move from their equilibrium position. All the atoms are displaced the same amount and still maintain their relative geometry. When the stresses are removed, all the atoms return to their original positions and no permanent deformation occurs. According to the Hooke’s law, the stress is proportional to the strain (in the elastic region), and the slope is Young’s modulus. Young’s modulus is equal to the longitudinal stress divided by the strain.
Hardness of Brass vs Steel and Stainless Steel
Brinell hardness of cartridge brass – UNS C26000 is approximately 100 MPa.
Brinell hardness of stainless steel – type 304 is approximately 201 MPa.
Brinell hardness of low-carbon steel is approximately 120 MPa.
Brinell hardness of high-carbon steel is approximately 200 MPa.
Rockwell hardness test is one of the most common indentation hardness tests, that has been developed for hardness testing. In contrast to Brinell test, the Rockwell tester measures the depth of penetration of an indenter under a large load (major load) compared to the penetration made by a preload (minor load). The minor load establishes the zero position. The major load is applied, then removed while still maintaining the minor load. The difference between depth of penetration before and after application of the major load is used to calculate the Rockwell hardness number. That is, the penetration depth and hardness are inversely proportional. The chief advantage of Rockwell hardness is its ability to display hardness values directly. The result is a dimensionless number noted as HRA, HRB, HRC, etc., where the last letter is the respective Rockwell scale.
The Rockwell C test is performed with a Brale penetrator (120°diamond cone) and a major load of 150kg.
Thermal Properties of Brass vs Steel and Stainless Steel
Thermal properties of materials refer to the response of materials to changes in their thermodynamics/thermodynamic-properties/what-is-temperature-physics/”>temperature and to the application of heat. As a solid absorbs thermodynamics/what-is-energy-physics/”>energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Brass vs Steel and Stainless Steel
Melting point of cartridge brass – UNS C26000 is around 950°C.
Melting point of stainless steel – type 304 steel is around 1450°C.
Melting point of low-carbon steel is around 1450°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Brass vs Steel and Stainless Steel
The thermal conductivity of cartridge brass – UNS C26000 is 120 W/(m.K).
The thermal conductivity of stainless steel – type 304 is 20 W/(m.K).
The thermal conductivity of typical steel is 20 W/(m.K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
We hope, this article, Brass vs Steel and Stainless Steel – Comparison – Pros and Cons, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.