As was written, a crystalline material is one in which the atoms are situated in a repeating or periodic array over large atomic distances—that is, long-range order exists, such that upon solidification, the atoms will position themselves in a repetitive three-dimensional pattern, in which each atom is bonded to its nearest neighbor atoms. But reality is different, real crystals are never perfect. There are always defects. The influence of these defects is not always adverse, and often specific characteristics are deliberately fashioned by the introduction of controlled amounts or numbers of particular defects.
Point Defects
Point defects have atomic dimensions, so that they occur only at or around a single lattice point. They are not extended in space in any dimension. Point imperfections in crystals can be divided into three main defect categories.
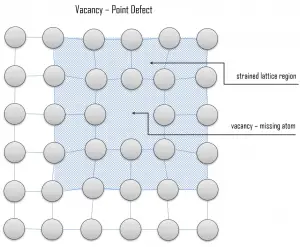 Vacancy defects. Vacancy defects result from a missing atom in a lattice position. The stability of the surrounding crystal structure guarantees that the neighboring atoms will not simply collapse around the vacancy. The vacancy type of defect can result from imperfect packing during the crystallization process, or it may be due to increased thermal vibrations of the atoms brought about by elevated temperature. All crystalline solids contain vacancies, and, in fact, it is not possible to create such a material that is free of these defects. A vacancy (or pair of vacancies in an ionic solid) is sometimes called a Schottky defect. This point defect forms when oppositely charged ions leave their lattice sites, creating vacancies. These vacancies are formed in stoichiometric units, to maintain an overall neutral charge in the ionic solid.
Vacancy defects. Vacancy defects result from a missing atom in a lattice position. The stability of the surrounding crystal structure guarantees that the neighboring atoms will not simply collapse around the vacancy. The vacancy type of defect can result from imperfect packing during the crystallization process, or it may be due to increased thermal vibrations of the atoms brought about by elevated temperature. All crystalline solids contain vacancies, and, in fact, it is not possible to create such a material that is free of these defects. A vacancy (or pair of vacancies in an ionic solid) is sometimes called a Schottky defect. This point defect forms when oppositely charged ions leave their lattice sites, creating vacancies. These vacancies are formed in stoichiometric units, to maintain an overall neutral charge in the ionic solid.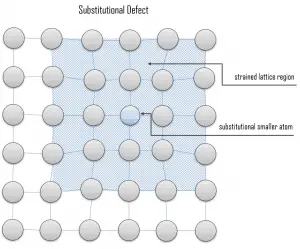 Substitutional defects. Due to fundamental limitations of material purification methods, materials are never 100% pure, which by definition induces defects in crystal structure. Substitutional defects result from an impurity present at a lattice position. For the substitutional type, solute or impurity atoms replace or substitute for the host atoms. Several features of the solute and solvent atoms determine the degree to which the former dissolves in the latter. These are expressed as the Hume–Rothery rules. According to these rules, substitutional solid solutions may form if the solute and solvent have:
Substitutional defects. Due to fundamental limitations of material purification methods, materials are never 100% pure, which by definition induces defects in crystal structure. Substitutional defects result from an impurity present at a lattice position. For the substitutional type, solute or impurity atoms replace or substitute for the host atoms. Several features of the solute and solvent atoms determine the degree to which the former dissolves in the latter. These are expressed as the Hume–Rothery rules. According to these rules, substitutional solid solutions may form if the solute and solvent have:
- Similar atomic radii (15% or less difference)
- Same crystal structure
- Similar electronegativities
- Similar valency a solid solution mixes with others to form a new solution
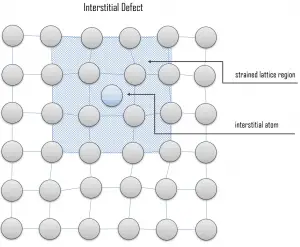 Interstitial defects. Interstitial defects result from an impurity located at an interstitial site or one of the lattice atoms being in an interstitial position instead of being at its lattice position. A self-interstitial is an atom from the crystal that is crowded into an interstitial site. In metals, a self-interstitial introduces relatively large distortions and stress in the surrounding lattice because the atom is substantially larger than the interstitial position in which it is situated. Interstitial defects are generally high energy configurations, on the other hand the formation of this defect is not highly probable. Small atoms (mostly impurities) in some crystals can occupy interstices without high energy, such as hydrogen.
Interstitial defects. Interstitial defects result from an impurity located at an interstitial site or one of the lattice atoms being in an interstitial position instead of being at its lattice position. A self-interstitial is an atom from the crystal that is crowded into an interstitial site. In metals, a self-interstitial introduces relatively large distortions and stress in the surrounding lattice because the atom is substantially larger than the interstitial position in which it is situated. Interstitial defects are generally high energy configurations, on the other hand the formation of this defect is not highly probable. Small atoms (mostly impurities) in some crystals can occupy interstices without high energy, such as hydrogen.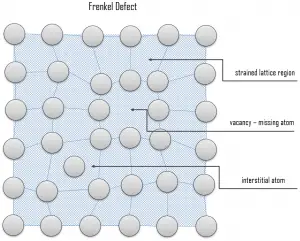 Frenkel Defects. A Frenkel defect or a Frenkel pair is a nearby pair of a vacancy and an interstitial defect. This is caused when an ion moves into an interstitial site and creates a vacancy. Their primary mechanism of generation is by particle irradiation. Frenkel defects are typical for radiation damage caused by high-energy neutrons. A 1 MeV neutron may affect approximately 5000 atoms. The presence of many displacement spikes will change the properties of the material being irradiated. A displacement spike contains large numbers of interstitials and lattice vacancies.
Frenkel Defects. A Frenkel defect or a Frenkel pair is a nearby pair of a vacancy and an interstitial defect. This is caused when an ion moves into an interstitial site and creates a vacancy. Their primary mechanism of generation is by particle irradiation. Frenkel defects are typical for radiation damage caused by high-energy neutrons. A 1 MeV neutron may affect approximately 5000 atoms. The presence of many displacement spikes will change the properties of the material being irradiated. A displacement spike contains large numbers of interstitials and lattice vacancies.
We hope, this article, Point Defects – Crystallographic Defects, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.