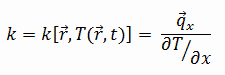Aluminium alloys are based on aluminium, in which the main alloying elements are Cu, Mn, Si, Mg, Mg+Si, Zn. Aluminium alloy compositions are registered with The Aluminum Association. The aluminium alloys are divided into 9 families (Al1xxx to Al9xxx). The different families of alloys and the major alloying elements are:
Aluminium alloys are based on aluminium, in which the main alloying elements are Cu, Mn, Si, Mg, Mg+Si, Zn. Aluminium alloy compositions are registered with The Aluminum Association. The aluminium alloys are divided into 9 families (Al1xxx to Al9xxx). The different families of alloys and the major alloying elements are:
- 1xxx: no alloying elements
- 2xxx: Copper
- 3xxx: Manganese
- 4xxx: Silicon
- 5xxx: Magnesium
- 6xxx: Magnesium and silicon
- 7xxx: Zinc, magnesium, and copper
- 8xxx: other elements which are not covered by other series
There are also two principal classifications, namely casting alloys and wrought alloys, both of which are further subdivided into the categories heat-treatable and non-heat-treatable. Aluminium alloys containing alloying elements with limited solid solubility at room temperature and with a strong temperature dependence of solid solubility (for example Cu) can be strengthened by a suitable thermal treatment (precipitation hardening). The strength of heat treated commercial Al alloys exceeds 550 MPa.
Mechanical properties of aluminium alloys highly depend on their phase composition and microstructure. High strength can be achieved among others by introduction of a high volume fraction of fine, homogeneously distributed second phase particles and by a refinement of the grain size. In general, aluminium alloys are characterized by a relatively low density (2.7 g/cm3 as compared to 7.9 g/cm3 for steel), high electrical and thermal conductivities, and a resistance to corrosion in some common environments, including the ambient atmosphere. The chief limitation of aluminum is its low melting temperature (660°C), which restricts the maximum temperature at which it can be used. For general production the 5000 and 6000 series alloys provide adequate strength combined with good corrosion resistance, high toughness and ease of welding.
Thermal Properties of Aluminium Alloys
Thermal properties of materials refer to the response of materials to changes in their thermodynamics/thermodynamic-properties/what-is-temperature-physics/”>temperature and to the application of heat. As a solid absorbs thermodynamics/what-is-energy-physics/”>energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Aluminium Alloys
Melting point of 2024 aluminium alloy is around 570°C.
Melting point of 6061 aluminium alloy is around 600°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Aluminium Alloys
The thermal conductivity of 2024 aluminium alloy is 140 W/(m.K).
The thermal conductivity of 6061 aluminium alloy is 150 W/(m.K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
We hope, this article, Thermal Properties of Aluminium Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.