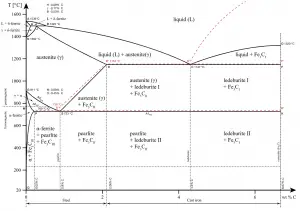
In materials engineering, cast irons are a class of ferrous alloys with carbon contents above 2.14 wt%. Typically, cast irons contain from 2.14 wt% to 4.0 wt% carbon and anywhere from 0.5 wt% to 3 wt% of silicon. Iron alloys with lower carbon content are known as steel. The difference is that cast irons can take advantage of eutectic solidification in the binary iron-carbon system. The term eutectic is Greek for “easy or well melting,” and the eutectic point represents the composition on the phase diagram where the lowest melting temperature is achieved. For the iron-carbon system the eutectic point occurs at a composition of 4.26 wt% C and a temperature of 1148°C.
Cast Iron Solidification
Cast iron is one of the most complex alloys used in industry. Because of the higher carbon content, the structure of cast iron, as opposed to that of steel, exhibits a carbon-rich phase. Depending primarily on composition, cooling rate, and melt treatment, the carbon-rich phase can solidify with formation of either a stable (austenite-graphite) or a metastable (austenite-Fe3C) eutectic. Cementite (Fe3C) is a metastable compound, and under some circumstances it can be made to dissociate or decompose to form α-ferrite and graphite, according to the reaction:
Fe3C → 3Fe (α) + C (graphite)
Thus, two types of eutectic solidification can occur. Moreover, various graphite shapes exist depending on chemical composition and cooling rate. Graphite formation is promoted by the presence of silicon in concentrations greater than about 1 wt%. Also, slower cooling rates during solidification favor graphitization (the formation of graphite).
We hope, this article, Cast Iron Solidification – Eutectic Point, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.