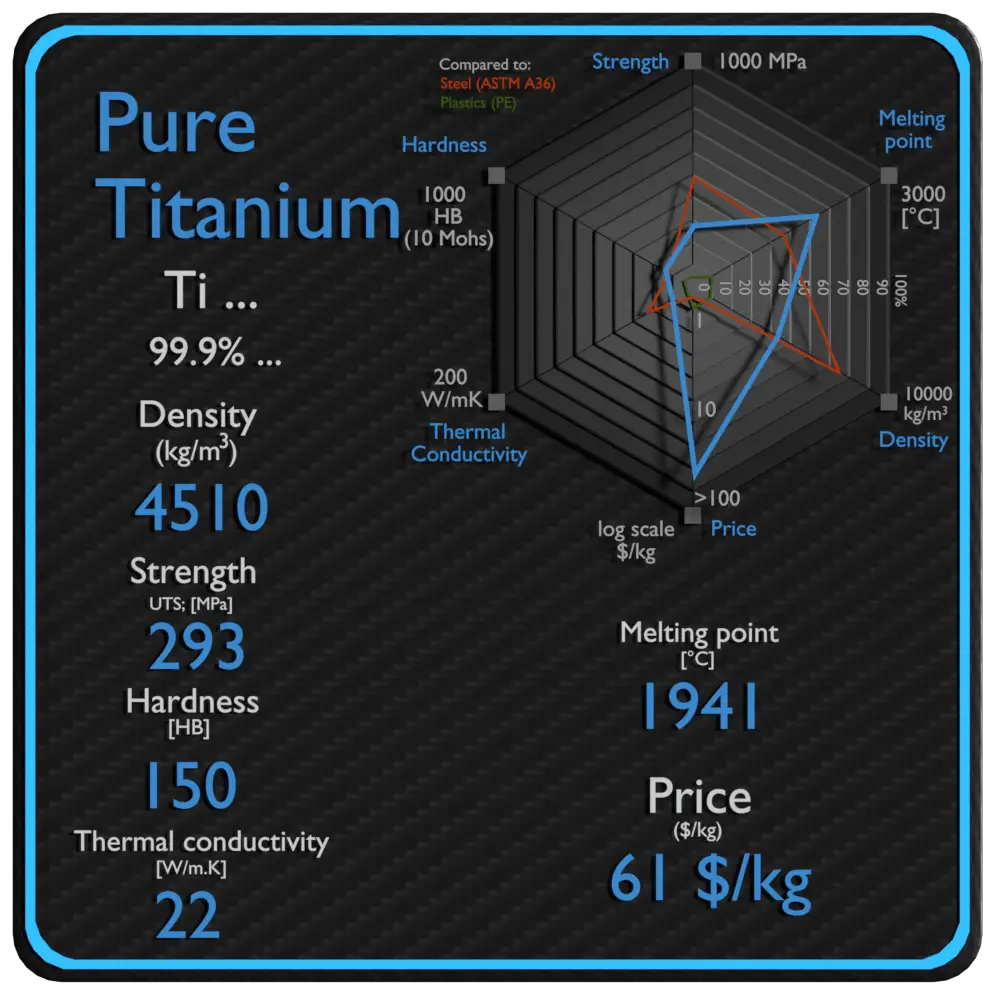
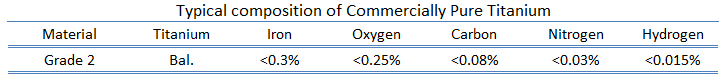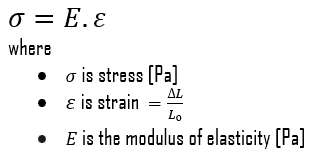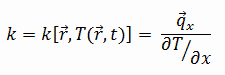Titanium is a lustrous transition metal with a silver color, low density, and high strength. Titanium is resistant to corrosion in sea water, aqua regia, and chlorine. In power plants, titanium can be used in surface condensers. The metal is extracted from its principal mineral ores by the Kroll and Hunter processes. Kroll’s process involved reduction of titanium tetrachloride (TiCl4), first with sodium and calcium, and later with magnesium, under an inert gas atmosphere. Pure titanium is stronger than common, low-carbon steels, but 45% lighter. It is also twice as strong as weak aluminium alloys but only 60% heavier. The two most useful properties of the metal are corrosion resistance and strength-to-density ratio, the highest of any metallic element. The corrosion resistance of titanium alloys at normal temperatures is unusually high. Titanium’s corrosion resistance is based on the formation of a stable, protective oxide layer. Although “commercially pure” titanium has acceptable mechanical properties and has been used for orthopedic and dental implants, for most applications titanium is alloyed with small amounts of aluminium and vanadium, typically 6% and 4% respectively, by weight. This mixture has a solid solubility which varies dramatically with temperature, allowing it to undergo precipitation strengthening.
Titanium alloys are metals that contain a mixture of titanium and other chemical elements. Such alloys have very high tensile strength and toughness (even at extreme temperatures). They are light in weight, have extraordinary corrosion resistance and the ability to withstand extreme temperatures.
Types of Titanium Alloys
Titanium exists in two crystallographic forms. At room temperature, unalloyed (commercially pure) titanium has a hexagonal close-packed (hcp) crystal structure referred to as alpha (α) phase. When the temperature of pure titanium reaches 885 °C (called the β transus temperature of titanium), the crystal structure changes to a bcc structure known as beta (β) phase. Alloying elements either raise or lower the temperature for the α-to- β transformation, so alloying elements in titanium are classified as either α stabilizers or β stabilizers. For example, vanadium, niobium, and molybdenum decrease the α-to-β transformation temperature and promote the formation of the β phase.
- Alpha Alloys. Alpha alloys contain elements such as aluminum and tin and are preferred for high temperature applications because of their superior creep characteristics.. These α-stabilizing elements work by either inhibiting change in the phase transformation temperature or by causing it to increase. The absence of a ductile-to-brittle transition, a feature of β alloys, makes α alloys suitable for cryogenic applications. On the other hand, cannot be strengthened by heat treatment because alpha is the stable phase and thus they are not so strength as beta alloys.
- Beta Alloys. Beta alloys contain transition elements such as vanadium, niobium, and molybdenum, which tend to decrease the temperature of the α to β phase transition. Beta alloys have excellent hardenability, and respond readily to heat treatment. These materials are highly forgeable and exhibit high fracture toughnesses. For example, ultimate tensile strength of high-strength titanium alloy – TI-10V-2Fe-3Al is about 1200 MPa.
- Alpha + Beta Alloy. Alpha + beta alloys have compositions that support a mixture of α and β phases and may contain between 10 and 50% β phase at room temperature. The most common α + β alloy is Ti-6Al-4V. The strength of these alloys may be improved and controlled by heat treatment. Examples include: Ti-6Al-4V, Ti-6Al-4V-ELI, Ti-6Al-6V-2Sn, Ti-6Al-7Nb.
Titanium Grade 2
Commercially pure titanium grade 2 is very similar to grade 1, but it has higher strength than grade 1 and excellent cold forming properties. It provides excellent welding properties and has excellent resistance to oxidation and corrosion. This grade of titanium is the most common grade of the commercially pure titanium industry.
Summary
| Name | Pure Titanium |
| Phase at STP | N/A |
| Density | 4510 kg/m3 |
| Ultimate Tensile Strength | 293 MPa |
| Yield Strength | 380 MPa |
| Young’s Modulus of Elasticity | 116 GPa |
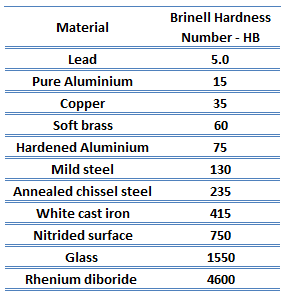
| Brinell Hardness | 150 BHN |
| Melting Point | 1941 °C |
| Thermal Conductivity | 22 W/mK |
| Heat Capacity | 520 J/g K |
| Price | 61 $/kg |
It is the prime choice for many fields of applications:
- Aerospace,
- Automotive,
- Chemical Processing & Chlorate Manufacturing,
- Desalination
- Power generation
Commercially Pure Titanium – Grade 1 in Steam Condensers
In nuclear power plants, the main steam condenser (MC) system is designed to condense and deaerate the exhaust steam from the main turbine and provide a heat sink for the turbine bypass system. The exhausted steam from the LP turbines is condensed by passing over tubes containing water from the cooling system. These tubes are usually made of stainless steel, copper alloys, or titanium depending on several selection criteria (such as thermal conductivity or corrosion resistance). Titanium condenser tubes are usually the best technical choice, however titanium is very expensive material and the use of titanium condenser tubes is associated with very high initial costs. Titanium in particular can bring major improvements such as higher water velocities promoting better heat coefficients, excellent resistance to abrasion, erosion and corrosion thereby improving resistance to fouling. Tubes are mostly welded tubes from ASTM SB 338 grade 1 made on a continuous manufacturing line. This commercially pure titanium is the softest titanium and has the highest ductility. It has good cold forming characteristics and provides excellent corrosion resistance. It also has excellent welding properties and high impact toughness. All manufacturing operations (welding, annealing, non-destructive testing) are fully automated to produce high quality tubes in large quantities.
Properties of Titanium Grade 2
Material properties are intensive properties, that means they are independent of the amount of mass and may vary from place to place within the system at any moment. The basis of materials science involves studying the structure of materials, and relating them to their properties (mechanical, electrical etc.). Once a materials scientist knows about this structure-property correlation, they can then go on to study the relative performance of a material in a given application. The major determinants of the structure of a material and thus of its properties are its constituent chemical elements and the way in which it has been processed into its final form.
Mechanical Properties of Titanium Grade 2
Materials are frequently chosen for various applications because they have desirable combinations of mechanical characteristics. For structural applications, material properties are crucial and engineers must take them into account.
Strength of Titanium Grade 2
In mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. Strength of materials basically considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. Strength of a material is its ability to withstand this applied load without failure or plastic deformation.
Ultimate Tensile Strength
Ultimate tensile strength of commercially pure titanium – Grade 2 is about 340 MPa.
 The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after the ultimate strength has been achieved. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for an aluminum to as high as 3000 MPa for very high-strength steels.
The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after the ultimate strength has been achieved. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for an aluminum to as high as 3000 MPa for very high-strength steels.
Yield Strength
Yield strength of commercially pure titanium – Grade 2 is about 300 MPa.
The yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning plastic behavior. Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically whereas yield point is the point where nonlinear (elastic + plastic) deformation begins. Prior to the yield point, the material will deform elastically and will return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible. Some steels and other materials exhibit a behaviour termed a yield point phenomenon. Yield strengths vary from 35 MPa for a low-strength aluminum to greater than 1400 MPa for very high-strength steels.
Young’s Modulus of Elasticity
Young’s modulus of elasticity of commercially pure titanium – Grade 2 is about 105 GPa.
The Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests. Up to a limiting stress, a body will be able to recover its dimensions on removal of the load. The applied stresses cause the atoms in a crystal to move from their equilibrium position. All the atoms are displaced the same amount and still maintain their relative geometry. When the stresses are removed, all the atoms return to their original positions and no permanent deformation occurs. According to the Hooke’s law, the stress is proportional to the strain (in the elastic region), and the slope is Young’s modulus. Young’s modulus is equal to the longitudinal stress divided by the strain.
Hardness of Titanium Grade 2
Rockwell hardness of commercially pure titanium – Grade 2 is approximately 80 HRB.
Rockwell hardness test is one of the most common indentation hardness tests, that has been developed for hardness testing. In contrast to Brinell test, the Rockwell tester measures the depth of penetration of an indenter under a large load (major load) compared to the penetration made by a preload (minor load). The minor load establishes the zero position. The major load is applied, then removed while still maintaining the minor load. The difference between depth of penetration before and after application of the major load is used to calculate the Rockwell hardness number. That is, the penetration depth and hardness are inversely proportional. The chief advantage of Rockwell hardness is its ability to display hardness values directly. The result is a dimensionless number noted as HRA, HRB, HRC, etc., where the last letter is the respective Rockwell scale.
The Rockwell C test is performed with a Brale penetrator (120°diamond cone) and a major load of 150kg.
Thermal Properties of Titanium Grade 2
Thermal properties of materials refer to the response of materials to changes in their thermodynamics/thermodynamic-properties/what-is-temperature-physics/”>temperature and to the application of heat. As a solid absorbs thermodynamics/what-is-energy-physics/”>energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Titanium Grade 2
Melting point of commercially pure titanium – Grade 2 is around 1660°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Titanium Grade 2
The thermal conductivity of commercially pure titanium – Grade 2 is 16 W/(m.K).
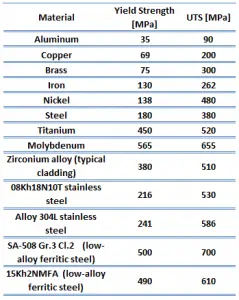
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
[/lgc_column]We hope, this article, Titanium Grade 2, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.