Quenching
The term quenching refers to a heat treatment in which a material is rapidly cooled in water, oil or air to obtain certain material properties, especially hardness. In ferrous alloys, quenching is most commonly used to harden steel by introducing martensite, while non-ferrous alloys will usually become softer than normal. Above this critical temperature, a metal is partially or fully austenitized, the cooling rate of the steel has to be rapid so as to let the austenite transform into metastable bainite or martensite.
The selection of a quenchant medium depends on the hardenability of the particular alloy, the section thickness and shape involved, and the cooling rates needed to achieve the desired micro-structure.
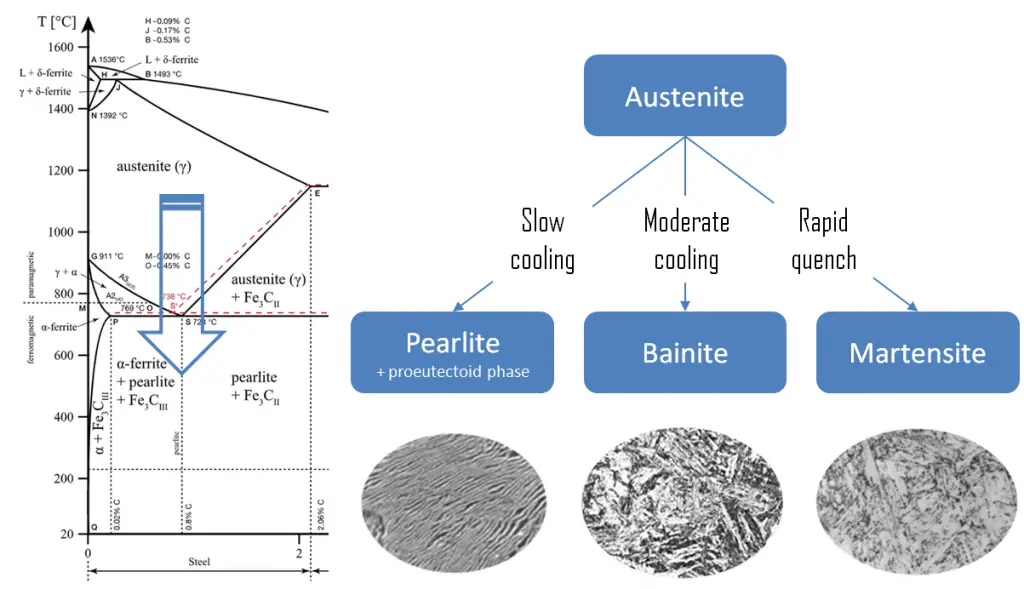 Martensite is a very hard metastable structure with a body-centered tetragonal (BCT) crystal structure. Martensite is formed in steels when the cooling rate from austenite is at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite (Fe3C). Therefore, it is a product of diffusionless transformation. Any diffusion whatsoever results in the formation of ferrite and cementite phases. It is named after the German metallurgist Adolf Martens (1850–1914).
Martensite is a very hard metastable structure with a body-centered tetragonal (BCT) crystal structure. Martensite is formed in steels when the cooling rate from austenite is at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite (Fe3C). Therefore, it is a product of diffusionless transformation. Any diffusion whatsoever results in the formation of ferrite and cementite phases. It is named after the German metallurgist Adolf Martens (1850–1914).
The microstructure of martensite in steels has different morphologies and may appear as either lath martensite or plate martensite. For steel 0–0.6% carbon the martensite has the appearance of lath, and is called lath martensite. For steel greater than 1% carbon it will form a plate like structure called plate martensite. Plate martensite, as the name indicates, forms as lenticular (lens-shaped) crystals with a zigzag pattern of smaller plates. Between those two percentages, the physical appearance of the grains is a mix of the two. The strength of the martensite is reduced as the amount of retained austenite grows.
Tempering
The term tempering refers to a heat treatment which is used to increase the toughness of iron-based alloys. Tempering is usually performed after hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical point for a certain period of time, then allowing it to cool in still air. Tempering makes the metal less hard while making it better able to sustain impacts without breaking. Tempering will cause the dissolved alloying elements to precipitate, or in the case of quenched steels, improve impact strength and ductile properties. Upon heating, the carbon atoms diffuse and react in a series of distinct steps that eventually form Fe3C or an alloy carbide in a ferrite matrix of gradually decreasing stress level.
For tempering, temperature is much more important than time at temperature. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, between 150 and 200 ° and maintain much of the hardness and strength of the quenched martensite and provide a small improvement in ductility and toughness. While springs are tempered at much higher temperatures. Tempering above 425 °C significantly improves ductility and toughness but at the expense of hardness and strength. Under certain conditions, hardness may remain unaffected by tempering or may even be increased as a result of it. Also, those alloy steels that contain one or more of the carbide-forming elements (chromium, molybdenum, vanadium, and tungsten) are capable of secondary hardening: they may become somewhat harder as a result of tempering.
Tempered Martensite
The relative ability of a ferrous alloy to form martensite is called hardenability. Hardenability is commonly measured as the distance below a quenched surface at which the metal exhibits a specific hardness of 50 HRC, for example, or a specific percentage of martensite in the microstructure. The highest hardness of a pearlitic steel is 43 HRC whereas martensite can achieve 72 HRC. Fresh martensite is very brittle if carbon content is greater than approximately 0.2 to 0.3%. It is so brittle that it cannot be used for most applications. This brittleness can be removed (with some loss of hardness) if the quenched steel is heated slightly in a process known as tempering. Tempering is accomplished by heating a martensitic steel to a temperature below the eutectoid for a specified time period (for example between 250°C and 650°C ).
This tempering heat treatment allows, by diffusional processes, the formation of tempered martensite, according to the reaction:
martensite (BCT, single phase) → tempered martensite (ferrite + Fe3C phases)
where the single-phase BCT martensite, which is supersaturated with carbon, transforms into the tempered martensite, composed of the stable ferrite and cementite phases. Its microstructure is similar to the microstructure of spheroidite but in this case tempered martensite contains extremely small and uniformly dispersed cementite particles embedded within a continuous ferrite matrix. Tempered martensite may be nearly as hard and strong as martensite but with substantially enhanced ductility and toughness.
We hope, this article, Quenching and Tempering, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.