The term quenching refers to a heat treatment in which a material is rapidly cooled in water, oil or air to obtain certain material properties, especially hardness. In ferrous alloys, quenching is most commonly used to harden steel by introducing martensite, while non-ferrous alloys will usually become softer than normal. Above this critical temperature, a metal is partially or fully austenitized, the cooling rate of the steel has to be rapid so as to let the austenite transform into metastable bainite or martensite.
The selection of a quenchant medium depends on the hardenability of the particular alloy, the section thickness and shape involved, and the cooling rates needed to achieve the desired micro-structure.
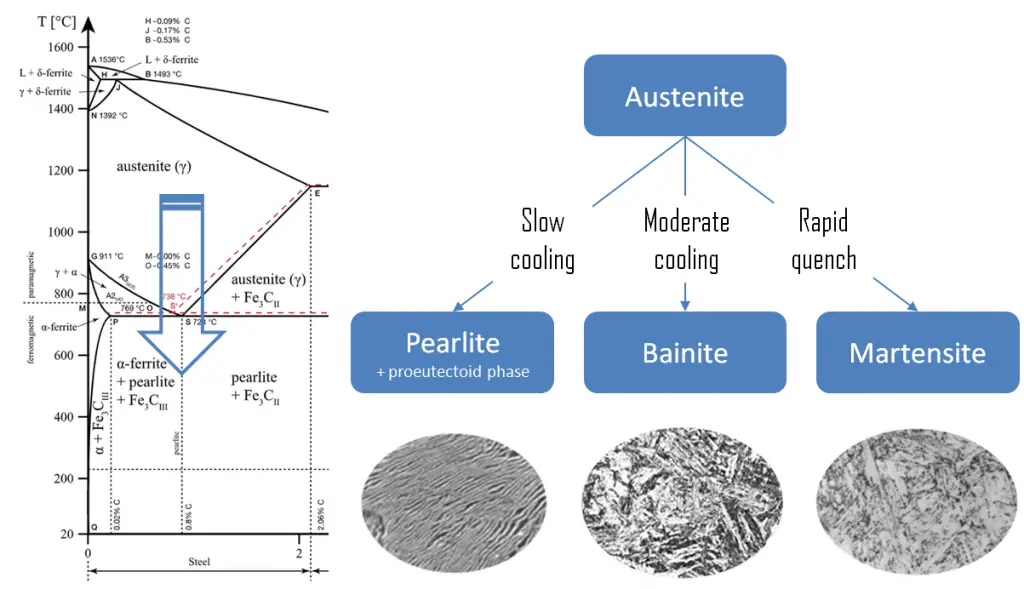 Martensite is a very hard metastable structure with a body-centered tetragonal (BCT) crystal structure. Martensite is formed in steels when the cooling rate from austenite is at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite (Fe3C). Therefore, it is a product of diffusionless transformation. Any diffusion whatsoever results in the formation of ferrite and cementite phases. It is named after the German metallurgist Adolf Martens (1850–1914).
Martensite is a very hard metastable structure with a body-centered tetragonal (BCT) crystal structure. Martensite is formed in steels when the cooling rate from austenite is at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite (Fe3C). Therefore, it is a product of diffusionless transformation. Any diffusion whatsoever results in the formation of ferrite and cementite phases. It is named after the German metallurgist Adolf Martens (1850–1914).
The microstructure of martensite in steels has different morphologies and may appear as either lath martensite or plate martensite. For steel 0–0.6% carbon the martensite has the appearance of lath, and is called lath martensite. For steel greater than 1% carbon it will form a plate like structure called plate martensite. Plate martensite, as the name indicates, forms as lenticular (lens-shaped) crystals with a zigzag pattern of smaller plates. Between those two percentages, the physical appearance of the grains is a mix of the two. The strength of the martensite is reduced as the amount of retained austenite grows.
Martensitic Transformation
Transformation hardening, known also as martensitic transformation hardening, is one of most common methods of hardening, which is primarily used for steels (i.e. carbon steels as well as stainless steels). The martensitic transformation is not, however, unique to iron–carbon alloys. It is found in other systems and is characterized, in part, by the diffusionless transformation.
Martensitic steels use predominantly higher levels of C and Mn along with heat treatment to increase strength. The finished product will have a duplex micro-structure of ferrite with varying levels of degenerate martensite. This allows for varying levels of strength. In metallurgy, quenching is most commonly used to harden steel by introducing martensite. There is a balance between hardness and toughness in any steel; the harder the steel, the less tough or impact-resistant it is, and the more impact-resistant it is, the less hard it is.
Martensite is produced from austenite as a result of the quenching, or another form of rapid cooling. Austenite in iron-carbon alloys is generally only present above the critical eutectoid temperature (723°C), and below 1500°C, depending on carbon content. In case of normal cooling rates, as the austenite cools, the carbon diffuses out of the austenite and forms carbon rich iron-carbide (cementite) and leaves behind carbon poor ferrite. Depending on alloy composition, a layering of ferrite and cementite, called pearlite, may form. But in case of rapid cooling, the carbon does not have time enough to diffuse and the transforms to a highly strained body-centered tetragonal form called martensite that is supersaturated with carbon. All the carbon atoms remain as interstitial impurities in martensite. The cooling rate determines the relative proportions of martensite, ferrite, and cementite, and therefore determines the mechanical properties of the resulting steel, such as hardness, tensile strength and toughness as well.
Example: Martensitic Stainless Steel
 Martensitic stainless steels are similar to ferritic steels in being based on chromium but have higher carbon levels up as high as 1%. They are sometimes classified as low-carbon and high-carbon martensitic stainless steels. They contain 12 to 14% chromium, 0.2 to 1% molybdenum, and no significant amount of nickel. Higher amounts of carbon allows them to be hardened and tempered much like carbon and low-alloy steels. They have moderate corrosion resistance, but are considered hard, strong, slightly brittle. They are magnetic and they can be nondestructively tested using the magnetic particle inspection method, unlike austenitic stainless steel. A common martensitic stainless is AISI 440C, which contains 16 to 18% chromium and 0.95 to 1.2% carbon. Grade 440C stainless steel is used in the following applications: gage blocks, cutlery, ball bearings and races, molds and dies, knives.
Martensitic stainless steels are similar to ferritic steels in being based on chromium but have higher carbon levels up as high as 1%. They are sometimes classified as low-carbon and high-carbon martensitic stainless steels. They contain 12 to 14% chromium, 0.2 to 1% molybdenum, and no significant amount of nickel. Higher amounts of carbon allows them to be hardened and tempered much like carbon and low-alloy steels. They have moderate corrosion resistance, but are considered hard, strong, slightly brittle. They are magnetic and they can be nondestructively tested using the magnetic particle inspection method, unlike austenitic stainless steel. A common martensitic stainless is AISI 440C, which contains 16 to 18% chromium and 0.95 to 1.2% carbon. Grade 440C stainless steel is used in the following applications: gage blocks, cutlery, ball bearings and races, molds and dies, knives.
Tempered Martensite
The relative ability of a ferrous alloy to form martensite is called hardenability. Hardenability is commonly measured as the distance below a quenched surface at which the metal exhibits a specific hardness of 50 HRC, for example, or a specific percentage of martensite in the microstructure. The highest hardness of a pearlitic steel is 43 HRC whereas martensite can achieve 72 HRC. Fresh martensite is very brittle if carbon content is greater than approximately 0.2 to 0.3%. It is so brittle that it cannot be used for most applications. This brittleness can be removed (with some loss of hardness) if the quenched steel is heated slightly in a process known as tempering. Tempering is accomplished by heating a martensitic steel to a temperature below the eutectoid for a specified time period (for example between 250°C and 650°C ).
This tempering heat treatment allows, by diffusional processes, the formation of tempered martensite, according to the reaction:
martensite (BCT, single phase) → tempered martensite (ferrite + Fe3C phases)
where the single-phase BCT martensite, which is supersaturated with carbon, transforms into the tempered martensite, composed of the stable ferrite and cementite phases. Its microstructure is similar to the microstructure of spheroidite but in this case tempered martensite contains extremely small and uniformly dispersed cementite particles embedded within a continuous ferrite matrix. Tempered martensite may be nearly as hard and strong as martensite but with substantially enhanced ductility and toughness.
We hope, this article, Quenched Steel – Tempered Steel, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.