Reactor core melt accident is an event or sequence of events that result in the melting of part of the fuel in the reactor core. Although this event is very unlikely, it cannot be ruled out. There are many and many barriers that have to be breached. Especially, common (usually 3×100%) failure of the Emergency Core Cooling System (ECCS) must occur after severe loss of coolant accident.
This type of accident is known under term a nuclear meltdown (core meltdown), but this is not officially defined by the International Atomic Energy Agency or by the Nuclear Regulatory Commission. The core melt accident is a severe nuclear reactor accident that results in core damage from overheating. It occurs when the heat generated by a nuclear reactor exceeds the heat removed by the cooling systems to the point where at least one nuclear fuel element exceeds its melting point. The heat causing the melting of a reactor may originate from the nuclear chain reaction, but more commonly decay heat of the fission products contained in the fuel rods is the primary heat source.
If the reactor core remains dry for a considerable length of time, the temperature of the fuel rods rises and may locally reach levels that cause significant and irreversible core degradation. The mechanisms of this degradation are both chemical and mechanical. Depending on the local temperature levels, degradation may result in more or less severe hydrogen production, fission product (FP) release, and molten corium formation and propagation towards the lower head.
Special reference: Nuclear Power Reactor Core Melt Accidents ISBN: 978-2-7598-1835-8, IRSN 2015.
Nuclear Fuel Melting
 The thermal conductivity of uranium dioxide is very low when compared with metal uranium, uranium nitride, uranium carbide and zirconium cladding material. The thermal conductivity is one of parameters, which determine the fuel centerline temperature. This low thermal conductivity can result in localised overheating in the fuel centerline and therefore this overheating must be avoided. Overheating of the fuel is prevented by maintaining the steady state peak linear heat rate (LHR) or the Heat Flux Hot Channel Factor – FQ(z) below the level at which fuel centerline melting occurs. Expansion of the fuel pellet upon centerline melting may cause the pellet to stress the cladding to the point of failure.
The thermal conductivity of uranium dioxide is very low when compared with metal uranium, uranium nitride, uranium carbide and zirconium cladding material. The thermal conductivity is one of parameters, which determine the fuel centerline temperature. This low thermal conductivity can result in localised overheating in the fuel centerline and therefore this overheating must be avoided. Overheating of the fuel is prevented by maintaining the steady state peak linear heat rate (LHR) or the Heat Flux Hot Channel Factor – FQ(z) below the level at which fuel centerline melting occurs. Expansion of the fuel pellet upon centerline melting may cause the pellet to stress the cladding to the point of failure.
Although the melting point of UO2 is over 2,800°C,fuel is usually operated at a much lower peak centerline temperatures (less than 1,400°C). This provides enough margin to fuel melting and to loss of fuel integrity. In general, fuel melting must be excluded for condition III and condition IV accidents as well. But the Fukushima Daiichi nuclear disaster in 2011 raises the safety problem of nuclear power plants to a new level in the world. It is difficult to predict these events and all other beyond design-basis accidents and prepare for them due to their extreme rarity. Under these infrequent circumstances the plant may be unable to operate safely. Reduction in the safety margin of a plant can cause a catastrophic failures such as meltdowns
In case of nuclear fuel melting, it is necessary to distinguish in which event the fuel melting temperature is reached. Fuel melting can occur:
- Slow fuel rod overpower. In the event of an increase in fuel overpower that is slow compared to the rate of heat transfer through the fuel, melting occurs only on a local scale.
- Loss of ultimate heat sink. In the event of loss of reactor coolant, the power of the rod decreases, the fuel temperature is only a few tens of degrees Celsius higher than the cladding temperature.
- RIA accidents. In these accidents, the large and rapid deposition of energy in the fuel can result in melting, fragmentation, and dispersal of fuel.
Corium
Corium, also called fuel-containing material (FCM), is a lava-like material created in the core of a nuclear reactor during a meltdown accident. It consists of:
- mixture of nuclear fuel and oxidized zirconium clad,
- fission products,
- control rods,
- structural materials from the affected parts of the reactor, products of their chemical reaction with air, water and steam,
- and, in the event that the reactor vessel is breached, molten concrete from the floor of the reactor room.
If the temperature reaches the melting point of UO2, a fuel degrades usually from the center of the core. Due to the formation of the eutectic liquids, the melting temperature may be several hundred degrees below that of the UO2 melting point (3100 K). Zirconium from fuel clad, together with other metals, reacts with water and produces zirconium dioxide and hydrogen. The production of hydrogen is a major danger in reactor accidents. As the eutectic molten mass increases, the corium pool may be formed and expands axially and radially in the core until it reaches either the baffle or the core support plate. At this moment, the corium flows into the lower head. The degradation may ultimately result in very different configurations in the core simultaneously, ranging from intact or barely degraded rods to the formation of a corium pool or a bed of debris.
In all cases, the corium gradually evaporates the water present in the lower head. If there is no additional water supply and the debris configuration is such that it cannot be cooled effectively, the materials’ temperature gradually rises until it reaches the melting point of the steel structures (plates, tubes, etc.) located in the lower head. In the case of adequate cooling of the corium, it can solidify and the damage is limited to the reactor itself. However, in the absence of adequate cooling, corium may melt through the reactor vessel and flow out or be ejected as a molten stream by the pressure inside the reactor vessel.
However, core reflooding may not be beneficial in all conditions. The following pheomena can occur during reflooding:
- massive steam generation, with hydrogen production and an increase in reactor
- coolant system pressure;
- steam explosion through corium-water interaction;
- continuation of core melt, despite water inflow;
- faster release of fission products.
In the event of reactor-vessel failure during a core melt accident, the corium resulting from this core melt and the melting of internal structures will pour onto the reactor pit basemat. The molten core-concrete interaction (MCCI) is treated as one of the important phenomena that may lead to the late containment failure by basemat penetration in a hypothetical severe accident of light water reactors (LWRs). The process is driven by the high initial temperature of the molten corium and the decay heat that is generated inside the melt by the radioactive decay of the fission products. Obviously, the progression of MCCI takes paramount importance and plays a key role to threaten the integrity of the containment, the last barrier of fission products.
In-vessel Retention
With regards to the safety of the Nuclear Power Plants (NPP) in case of a severe nuclear accident, one of the main challenges associated is the retention of the molten nuclear fuel and reactor internals, called corium, within the Reactor Pressure Vessel (RPV). One of the ways of cooling corium with in the RPV is by cooling the vessel from outside. The in-vessel retention can be achieved by full flooding of reactor cavity to cool the external wall of the lower head thereby avoiding structural failure by creep rupture. This strategy is termed as In-Vessel Retention (IVR). In case of the In-Vessel Retention (IVR) strategy, it is expected that the corium pool will be surrounded by an oxide crust, which will be in contact with molten steel from top of the pool as well as from sides of the vessel. Application of this approach to large power reactors is not trivial because of relatively short time between the detection of core melting and the lower head failure.
High Temperature Steam Oxidation of Zirconium Alloys
At high temperatures, the exothermic reaction of Zr-base alloys with steam is much more intensive and hazardous for the safety of nuclear power plants during accidents like a loss-of-coolant accident (LOCA). The main problem of high temperature oxidation is that zirconium cladding rapidly reacts with water steam at high temperature. The oxidation kinetics of relevant zirconium alloys appears to be parabolic in the temperature range of 1000-1500°C for many Zr-based alloys. Above 1577°C, the oxide layer transforms from tetragonal to cubic and the oxidation rate even increases. Moreover, oxidation of zirconium by water is accompanied by release of hydrogen gas. This oxidation is accelerated at high temperatures, e.g. inside a reactor core if the fuel assemblies are no longer completely covered by liquid water and insufficiently cooled. Metallic zirconium is then oxidized by water/steam to form hydrogen gas according to the following redox reaction:
Zr + 2H2O→ZrO2 + 2H2 (Q = 190 kJ/mol; Baker and Just)
See also: High Temperature Steam Oxidation of Zirconium Alloys
Containment Building
The containment building is primarily designed to prevent or mitigate the uncontrolled release of radioactive material to the environment in operational states and in accident conditions. Therefore it is considered to be the fourth and final barrier in the Defence in depth strategy.
While the containment plays a crucial role in Design Basis Accidents or in Design Extension conditions, it is “only” designed to condense steam from primary coolant and to contain it inside the building.
In case of Design Basis Accidents such as the Large Break Loss of Coolant Accident (LBLOCA) the pressure increase is usually significant and active containment systems (pressure-suppression systems) must be available in order to maintain the integrity (to keep the pressure and temperature under certain limits) of the containment building.
See also: Containment Building
See also: Hydrogen Mitigation

 The thermal conductivity of uranium dioxide is very low when compared with metal uranium, uranium nitride, uranium carbide and zirconium cladding material. The thermal conductivity is one of parameters, which determine the fuel centerline temperature. This low thermal conductivity can result in localised overheating in the fuel centerline and therefore this overheating must be avoided. Overheating of the fuel is prevented by maintaining the steady state peak linear heat rate (LHR) or the
The thermal conductivity of uranium dioxide is very low when compared with metal uranium, uranium nitride, uranium carbide and zirconium cladding material. The thermal conductivity is one of parameters, which determine the fuel centerline temperature. This low thermal conductivity can result in localised overheating in the fuel centerline and therefore this overheating must be avoided. Overheating of the fuel is prevented by maintaining the steady state peak linear heat rate (LHR) or the 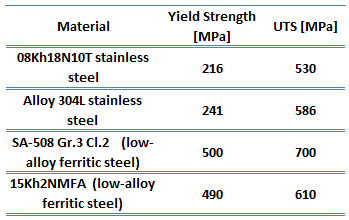 Low-carbon steel, also known as mild steel is now the most common form of
Low-carbon steel, also known as mild steel is now the most common form of 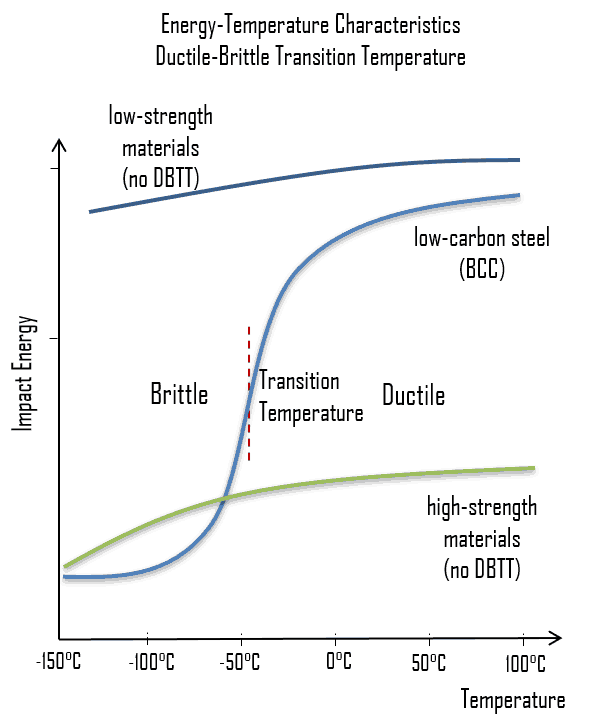 As was written, the distinction between brittleness and
As was written, the distinction between brittleness and