Materials Science
What is material?
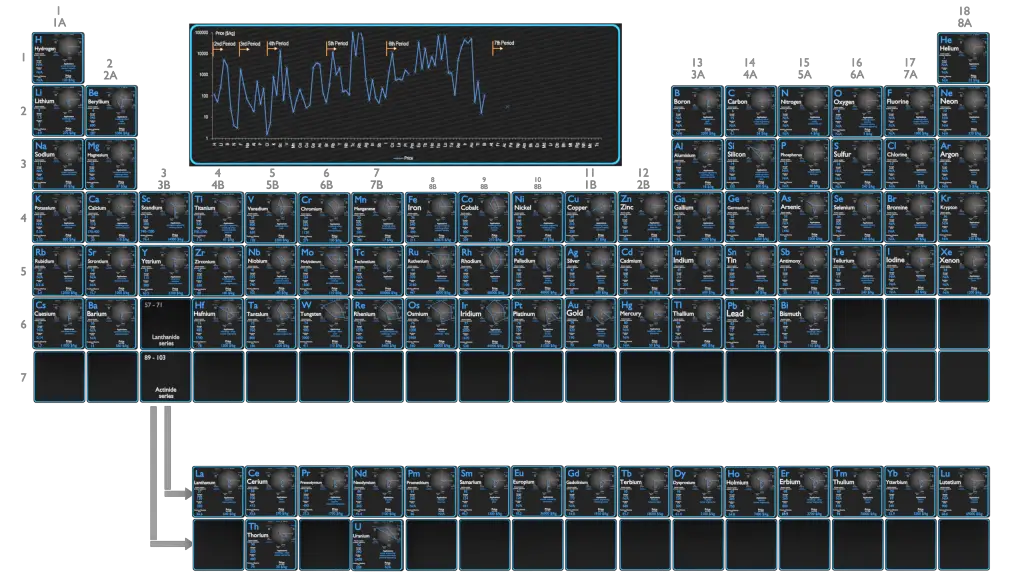
A material is defined as a substance (most often a solid, but other condensed phases can be included) that is intended to be used for certain applications. There are a myriad of materials around us – they can be found in anything from buildings to spacecraft.
Key Facts
- On the basis of chemistry and atomic structure, materials are classified into three general categories:
- Metals (metallic elements),
- Ceramics (compounds between metallic and nonmetallic elements),
- Polymers (compounds composed of carbon, hydrogen, and other nonmetallic elements).
- Real materials are never perfect. Classification of crystallographic defects (microscopic defects) is frequently made according to the geometry or dimensionality of the defect.
- Key mechanical design properties are:
- Stiffness. Stiffness is the ability of an object to resist deformation in response to an applied force.
- Strength. Strength is the ability of a material to resist deformation.
- Hardness. Hardness is the ability to withstand surface indentation and scratching.
- Ductility. Ductility is the ability of a material to deform under tensile load (% elongation).
- Toughness. Toughness is the ability of a material to absorb energy (or withstand shock) and plastically deform without fracturing.
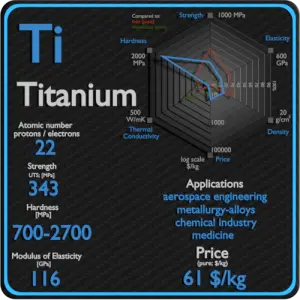
- Metal is a material (usually solid) comprising one or more metallic elements (e.g., iron, aluminium, copper, chromium, titanium, gold, nickel).
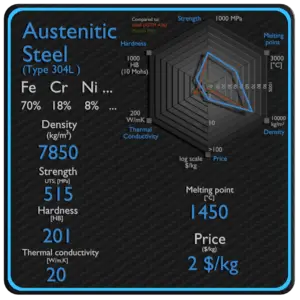
- Steels are iron–carbon alloys that may contain appreciable concentrations of other alloying elements. Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater strength.
- An alloy is a mixture of two or more materials, at least one of which is a metal. Alloys can have a microstructure consisting of solid solutions, where secondary atoms are introduced as substitutionals or interstitials in a crystal lattice.
- Non-destructive testing, NDT, is a very broad group of structural or material inspections and as the name implies, these inspections do not destroy the material/structure being examined.
Basis of Materials Science
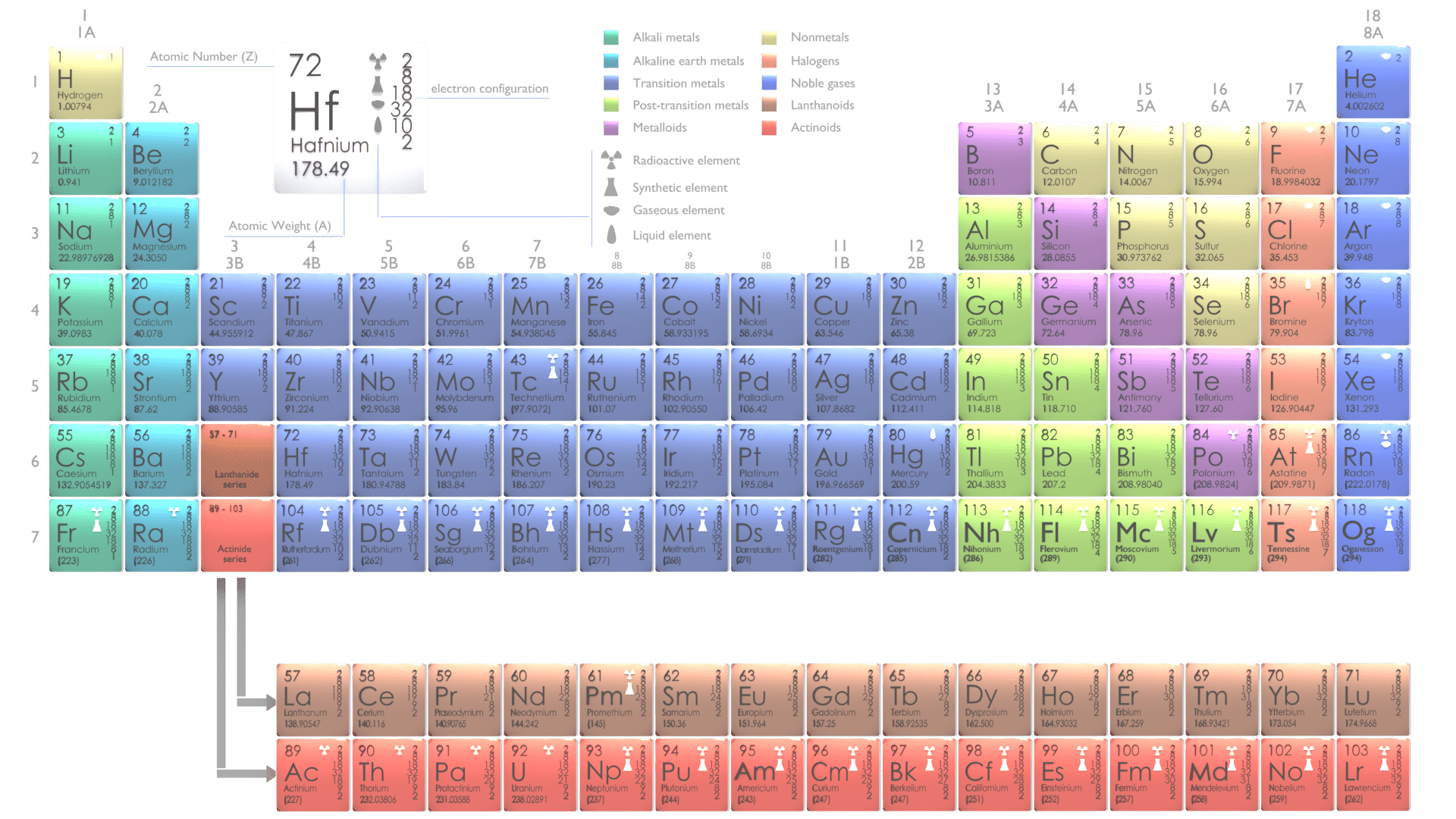
What are atoms?
The atoms are defined as the smallest constituents of ordinary matter, which can be divided without the release of electrically charged particles. The atoms consist of two parts. An atomic nucleus and an electron cloud.
Key Facts
- The physical world is composed of combinations of various subatomic or fundamental particles. These are the smallest building blocks of matter.
- The atoms consist of two parts. An atomic nucleus and an electron cloud.
- The number of electrons and their arrangement in the electron cloud is responsible for the chemical behavior of atoms.
- The nuclear properties (atomic mass, nuclear cross-sections) of the element are determined by the number of protons (atomic number) and number of neutrons (neutron number).
- Nuclear stability is a concept that helps to identify the stability of an isotope. To identify the stability of an isotope it is needed to find the ratio of neutrons to protons. To determine the stability of an isotope you can use the ratio neutron/proton (N/Z).
- There are only certain combinations of neutrons and protons, which forms stable nuclei.
- Unstable nuclei must undergo nuclear decay (radioactive decay) to stabilize itself, it is a random and natural process.
- The number of atoms in 1 mole (e.g. 12 grams of carbon) of a substance is equal to the Avogadro’s constant, which is equal to 6.022 x 1023.
About Materials Properties
Materials science and engineering is interdisciplinar and very important branch of study, which deals with the design and discovery of new materials, particularly solids. Materials science is one of the oldest forms of engineering and applied science and The material of choice of a given era is often a defining point (e.g. Stone Age, Bronze Age, Iron Age). The intellectual origins of materials science stem from the Enlightenment, when researchers began to use analytical thinking from chemistry, physics, and engineering to understand ancient, phenomenological observations in metallurgy and mineralogy. Sometimes it is useful to subdivide the discipline of materials science and engineering into materials science and materials engineering subdisciplines. The discipline of materials science involves investigating the relationships that exist between the structures and properties of materials. In contrast, materials engineering is, on the basis of these structure–property correlations, designing or engineering the structure of a material to produce a predetermined set of properties. Material properties are intensive properties, that means they are independent of the amount of mass and may vary from place to place within the system at any moment. The basis of materials science involves studying the structure of materials, and relating them to their properties (mechanical, electrical etc.). Once a materials scientist knows about this structure-property correlation, they can then go on to study the relative performance of a material in a given application. The major determinants of the structure of a material and thus of its properties are its constituent chemical elements and the way in which it has been processed into its final form.
About This Project
Main purpose of this project is to help the public to learn some interesting and important information about chemical elements and many common materials. We realize that the basics in the materials science can help people to understand many common problems. Anyone can be able to come here, learn the basics of materials science, material properties and to compare these properties. Feel free to ask a question, leave feedback or take a look at one of our articles.