An understanding of material science is essential for power plant personnel to understand why a material was selected for certain applications within their facility. Almost all processes that take place in the nuclear facilities involve the use of specialized metals. A basic understanding of material science is necessary for nuclear facility operators, maintenance personnel, and the technical staff to safely operate and maintain the facility and facility support systems. Our goal here will be to introduce material engineering of nuclear reactors. The knowledge of thermophysical and nuclear properties of materials is essential for designing nuclear power plants.
Classification of Materials
A material is defined as a substance (most often a solid, but other condensed phases can be included) that is intended to be used for certain applications. There are a myriad of materials around us – they can be found in anything from buildings to spacecraft. On the basis of chemistry and atomic structure, materials are classified into three general categories:
-
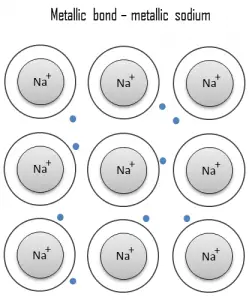
Metallic bond Metals. Metal is a material (usually solid) comprising one or more metallic elements (e.g., iron, aluminum, copper, chromium, titanium, gold, nickel), and often also nonmetallic elements (e.g., carbon, nitrogen, oxygen) in relatively small amounts. The unique feature of metals as far as their structure is concerned is the presence of charge carriers, specifically electrons. This feature is given by the nature of metallic bond. In metallic bond, the atoms do not share or exchange electrons to bond together. Instead, many electrons (roughly one for each atom) are more or less free to move throughout the metal, so that each electron can interact with many of the fixed atoms. The electrical and thermal conductivities of metals originate from the fact that their outer electrons are delocalized.
- Ceramics. A ceramic is a solid material comprising an inorganic compound of metal, non-metal or metalloid atoms primarily held in ionic and covalent bonds. Common examples are earthenware, porcelain, and brick. In nuclear industry, uranium dioxide is a ceramic refractory uranium compound, in many cases used as a nuclear fuel.
- Polymers. Polymers are compounds (macromolecules) composed of carbon, hydrogen, and other nonmetallic elements. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Some common and familiar polymers are polyethylene (PE), nylon, polycarbonate (PC), polystyrene (PS), and silicone rubber.
In addition, composites are composed of at least two different material types. Another materials category is the advanced materials that are used in high-tech applications, including:
- Semiconductors. In general, semiconductors are materials, inorganic or organic, which have the ability to control their conduction depending on chemical structure, temperature, illumination, and presence of dopants. The name semiconductor comes from the fact that these materials have an electrical conductivity between that of a metal, like copper, gold, etc. and an insulator, such as glass.
- Biomaterials. In general, a biomaterial is any substance that has been engineered to interact with biological systems for a medical purpose. These materials must not produce toxic substances and must be compatible with body tissues (i.e., must not cause adverse biological reactions).
- Smart materials. Smart materials are materials that sense and respond to changes in their environments in predetermined manners.
- Nanomaterials are materials that have structural features on the order of a nanometer, some of which may be designed on the atomic/molecular level.
We hope, this article, Classification of Materials, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.