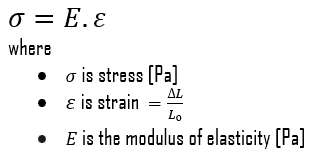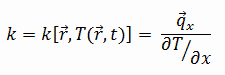Copper alloys are alloys based on copper, in which the main alloying elements are Zn, Sn, Si, Al, Ni. Cu-based alloys constitute mostly substitutional solid solutions, for which solute or impurity atoms replace or substitute for the host atoms. Several features of the solute and solvent atoms determine the degree to which the former dissolves in the latter. These are expressed as the Hume–Rothery rules. There are as many as 400 different copper and copper alloy compositions loosely grouped into the categories: copper, high copper alloy, brasses, bronzes, copper nickels, copper–nickel–zinc (nickel silver), leaded copper, and special alloys. In addition, a limited number of copper alloys can be strengthened by heat treatment.; consequently, cold working and/or solid-solution alloying must be used to improve these mechanical properties.
Copper alloys are alloys based on copper, in which the main alloying elements are Zn, Sn, Si, Al, Ni. Cu-based alloys constitute mostly substitutional solid solutions, for which solute or impurity atoms replace or substitute for the host atoms. Several features of the solute and solvent atoms determine the degree to which the former dissolves in the latter. These are expressed as the Hume–Rothery rules. There are as many as 400 different copper and copper alloy compositions loosely grouped into the categories: copper, high copper alloy, brasses, bronzes, copper nickels, copper–nickel–zinc (nickel silver), leaded copper, and special alloys. In addition, a limited number of copper alloys can be strengthened by heat treatment.; consequently, cold working and/or solid-solution alloying must be used to improve these mechanical properties.
Properties of Copper
Copper is a soft, tough, ductile and malleable material. These properties make copper extremely suitable for tube forming, wire drawing, spinning and deep drawing. The other key properties exhibited by copper and its alloys include:
- Excellent thermal conductivity. Copper has a 60% higher thermal conductivity rating than aluminium, so it is better able to reduce thermal hot spots in electrical wiring systems. The electrical and thermal conductivities of metals originate from the fact that their outer electrons are delocalized.
- Excellent electrical conductivity. The conductivity of copper is 97% that of silver. Due to its much lower cost and greater abundance, copper has traditionally been the standard material used for electricity transmission applications. However, aluminium is usually used in overhead high-voltage power lines because it has about half the weight and lower cost of a comparable resistance copper cable. At a given temperature, the thermal and electrical conductivities of metals are proportional, but raising the temperature increases the thermal conductivity while decreasing the electrical conductivity. This behavior is quantified in the Wiedemann–Franz law.
- Good corrosion resistance. Copper does not react with water, but it does slowly react with atmospheric oxygen to form a layer of brown-black copper oxide which, unlike the rust that forms on iron in moist air, protects the underlying metal from further corrosion (passivation). Copper nickel alloys, aluminium brass, and aluminium demonstrate superior resistance to saltwater corrosion.
- Good biofouling resistance
- Good machinability. Machining of copper is possible, although alloys are preferred for good machinability in creating intricate parts.
- Retention of mechanical and electrical properties at cryogenic temperatures
- Diamagnetic
Properties of Copper Alloys
Material properties are intensive properties, that means they are independent of the amount of mass and may vary from place to place within the system at any moment. The basis of materials science involves studying the structure of materials, and relating them to their properties (mechanical, electrical etc.). Once a materials scientist knows about this structure-property correlation, they can then go on to study the relative performance of a material in a given application. The major determinants of the structure of a material and thus of its properties are its constituent chemical elements and the way in which it has been processed into its final form.
Mechanical Properties of Copper Alloys
Materials are frequently chosen for various applications because they have desirable combinations of mechanical characteristics. For structural applications, material properties are crucial and engineers must take them into account.
Strength of Copper Alloys
In mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. Strength of materials basically considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. Strength of a material is its ability to withstand this applied load without failure or plastic deformation.
Ultimate Tensile Strength
Ultimate tensile strength of electrolytic-tough pitch (ETP) copper it is about 250 MPa.
Ultimate tensile strength of carthridge brass – UNS C26000 is about 315 MPa.
Ultimate tensile strength of aluminium bronze – UNS C95400 is about 550 MPa.
Ultimate tensile strength of tin bronze – UNS C90500 – gun metal is about 310 MPa.
Ultimate tensile strength of copper beryllium – UNS C17200 is about 1380 MPa.
Ultimate tensile strength of cupronickel – UNS C70600 is about 275 MPa.
Ultimate tensile strength of nickel silver – UNS C75700 is about 400 MPa.
 The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after the ultimate strength has been achieved. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for an aluminum to as high as 3000 MPa for very high-strength steels.
The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after the ultimate strength has been achieved. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for an aluminum to as high as 3000 MPa for very high-strength steels.
Yield Strength
Proof strength of electrolytic-tough pitch (ETP) copper is between 60-300 MPa.
Yield strength of aluminium bronze – UNS C95400 is about 250 MPa.
Yield strength of tin bronze – UNS C90500 – gun metal is about 150 MPa.
Yield strength of copper beryllium – UNS C17200 is about 1100 MPa.
Yield strength of cupronickel – UNS C70600 is about 105 MPa.
Yield strength of nickel silver – UNS C75700 is about 170 MPa.
The yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning plastic behavior. Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically whereas yield point is the point where nonlinear (elastic + plastic) deformation begins. Prior to the yield point, the material will deform elastically and will return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible. Some steels and other materials exhibit a behaviour termed a yield point phenomenon. Yield strengths vary from 35 MPa for a low-strength aluminum to greater than 1400 MPa for very high-strength steels.
Young’s Modulus of Elasticity
Young’s modulus of elasticity of electrolytic-tough pitch (ETP) copper is about 120 GPa.
Young’s modulus of elasticity of carthridge brass – UNS C26000 is about 95 GPa.
Young’s modulus of elasticity of aluminium bronze – UNS C95400 is about 110 GPa.
Young’s modulus of elasticity of tin bronze – UNS C90500 – gun metal is about 103 GPa.
Young’s modulus of elasticity of copper beryllium – UNS C17200 is about 131 GPa.
Young’s modulus of elasticity of cupronickel – UNS C70600 is about 135 GPa.
Young’s modulus of elasticity of nickel silver – UNS C75700 is about 117 GPa.
The Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests. Up to a limiting stress, a body will be able to recover its dimensions on removal of the load. The applied stresses cause the atoms in a crystal to move from their equilibrium position. All the atoms are displaced the same amount and still maintain their relative geometry. When the stresses are removed, all the atoms return to their original positions and no permanent deformation occurs. According to the Hooke’s law, the stress is proportional to the strain (in the elastic region), and the slope is Young’s modulus. Young’s modulus is equal to the longitudinal stress divided by the strain.
Hardness of Copper Alloys
Vickers hardness of electrolytic-tough pitch (ETP) copper depends greatly on the temper of the material, but it is between 50 – 150 HV.
Brinell hardness of carthridge brass – UNS C26000 is approximately 100 MPa.
Brinell hardness of aluminium bronze – UNS C95400 is approximately 170 MPa. The hardness of aluminum bronzes increases with aluminum (and other alloy) content as well as with stresses caused through cold working.
Brinell hardness of tin bronze – UNS C90500 – gun metal is approximately 75 BHN.
Rockwell hardness of copper beryllium – UNS C17200 is approximately 82 HRB.
Brinell hardness of cupronickel – UNS C70600 is approximately HB 100.
Rockwell hardness of nickel silver – UNS C75700 is approximately 45 HRB.
Rockwell hardness test is one of the most common indentation hardness tests, that has been developed for hardness testing. In contrast to Brinell test, the Rockwell tester measures the depth of penetration of an indenter under a large load (major load) compared to the penetration made by a preload (minor load). The minor load establishes the zero position. The major load is applied, then removed while still maintaining the minor load. The difference between depth of penetration before and after application of the major load is used to calculate the Rockwell hardness number. That is, the penetration depth and hardness are inversely proportional. The chief advantage of Rockwell hardness is its ability to display hardness values directly. The result is a dimensionless number noted as HRA, HRB, HRC, etc., where the last letter is the respective Rockwell scale.
The Rockwell C test is performed with a Brale penetrator (120°diamond cone) and a major load of 150kg.
Thermal Properties of Copper Alloys
Thermal properties of materials refer to the response of materials to changes in their thermodynamics/thermodynamic-properties/what-is-temperature-physics/”>temperature and to the application of heat. As a solid absorbs thermodynamics/what-is-energy-physics/”>energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Copper Alloys
Melting point of electrolytic-tough pitch (ETP) copper is around 1085°C.
Melting point of carthridge brass – UNS C26000 is around 950°C.
Melting point of aluminium bronze – UNS C95400 is around 1030°C.
Melting point of tin bronze – UNS C90500 – gun metal is around 1000°C.
Melting point of copper beryllium – UNS C17200 is around 866°C.
Melting point of cupronickel – UNS C70600 is around 1100°C.
Melting point of nickel silver – UNS C75700 is around 1040°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Copper Alloys
The thermal conductivity of electrolytic-tough pitch (ETP) copper is 394 W/(m.K).
The thermal conductivity of carthridge brass – UNS C26000 is 120 W/(m.K).
The thermal conductivity of aluminium bronze – UNS C95400 is 59 W/(m.K).
The thermal conductivity of tin bronze – UNS C90500 – gun metal is 75 W/(m.K).
The thermal conductivity of copper beryllium – UNS C17200 is 115 W/(m.K).
The thermal conductivity of cupronickel – UNS C70600 is 40 W/(m.K).
The thermal conductivity of nickel silver – UNS C75700 is 40 W/(m.K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
Electrical Conductivity of Copper Alloys
The electrical conductivity of electrolytic-tough pitch (ETP) copper is 101% IACS (around 58.6 MS/m).
The electrical conductivity of carthridge brass – UNS C26000 is about 30% IACS (around 17 MS/m).
Electrical resistivity and its converse, electrical conductivity, is a fundamental property of a material that quantifies how strongly it resists or conducts the flow of electric current. A low resistivity indicates a material that readily allows the flow of electric current. The symbol of resistivity is usually the Greek letter ρ (rho). The SI unit of electrical resistivity is the ohm-metre (Ω⋅m). Note that, electrical resistivity is not the same as electrical resistance. Electrical resistance is expressed in Ohms. While resistivity is a material property, resistance is the property of an object.
We hope, this article, Properties of Copper Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.