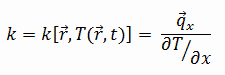Brass is is the generic term for a range of copper-zinc alloys. Brass can be alloyed with zinc in different proportions, which results in a material of varying mechanical, corrosion and thermal properties. Increased amounts of zinc provide the material with improved strength and ductility. Brasses with a copper content greater than 63% are the most ductile of any copper alloy and are shaped by complex cold forming operations. Brass has higher malleability than bronze or zinc. The relatively low melting point of brass and its fluidity make it a relatively easy material to cast. Brass can range in surface color from red to yellow to gold to silver depending on the zinc content. Some of the common uses for brass alloys include costume jewelry, locks, hinges, gears, bearings, hose couplings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins. Brass and bronze are common engineering materials in modern architecture and primarily used for roofing and facade cladding due to their visual appearance.
Brass is is the generic term for a range of copper-zinc alloys. Brass can be alloyed with zinc in different proportions, which results in a material of varying mechanical, corrosion and thermal properties. Increased amounts of zinc provide the material with improved strength and ductility. Brasses with a copper content greater than 63% are the most ductile of any copper alloy and are shaped by complex cold forming operations. Brass has higher malleability than bronze or zinc. The relatively low melting point of brass and its fluidity make it a relatively easy material to cast. Brass can range in surface color from red to yellow to gold to silver depending on the zinc content. Some of the common uses for brass alloys include costume jewelry, locks, hinges, gears, bearings, hose couplings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins. Brass and bronze are common engineering materials in modern architecture and primarily used for roofing and facade cladding due to their visual appearance.
For example, UNS C26000 cartridge brass alloy (70/30) is from the yellow brass series, which has the highest ductility. Cartridge brasses are mostly cold formed and they can also be easily machined, which is necessary in making cartridge cases. It can be used for radiator cores and tanks, flashlight shells, lamp fixtures, fasteners, locks, hinges, ammunition components or plumbing accessories.
Thermal Properties of Brass – Cartridge Brass – UNS C26000
Thermal properties of materials refer to the response of materials to changes in their thermodynamics/thermodynamic-properties/what-is-temperature-physics/”>temperature and to the application of heat. As a solid absorbs thermodynamics/what-is-energy-physics/”>energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Brass – Cartridge Brass – UNS C26000
Melting point of cartridge brass – UNS C26000 is around 950°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Brass – Cartridge Brass – UNS C26000
The thermal conductivity of cartridge brass – UNS C26000 is 120 W/(m.K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
We hope, this article, Thermal Properties of Brass – Melting Point – Conductivity, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.