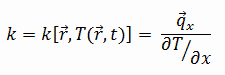Magnesium alloys are mixtures of magnesium and other alloying metal, usually aluminium, zinc, silicon, manganese, copper and zirconium. Since the most outstanding characteristic of magnesium is its density, 1.7 g/cm3, its alloys are used where light weight is an important consideration (e.g., in aircraft components). Magnesium has the lowest melting point (923 K (1,202 °F)) of all the alkaline earth metals. Pure magnesium has an HCP crystal structure, is relatively soft, and has a low elastic modulus: 45 GPa. Magnesium alloys have also a hexagonal lattice structure, which affects the fundamental properties of these alloys. At room temperature, magnesium and its alloys are difficult to perform cold working due to the fact plastic deformation of the hexagonal lattice is more complicated than in cubic latticed metals like aluminium, copper and steel. Therefore, magnesium alloys are typically used as cast alloys. Despite the reactive nature of the pure magnesium powder, magnesium metal and its alloys have good resistance to corrosion.
Magnesium alloys are mixtures of magnesium and other alloying metal, usually aluminium, zinc, silicon, manganese, copper and zirconium. Since the most outstanding characteristic of magnesium is its density, 1.7 g/cm3, its alloys are used where light weight is an important consideration (e.g., in aircraft components). Magnesium has the lowest melting point (923 K (1,202 °F)) of all the alkaline earth metals. Pure magnesium has an HCP crystal structure, is relatively soft, and has a low elastic modulus: 45 GPa. Magnesium alloys have also a hexagonal lattice structure, which affects the fundamental properties of these alloys. At room temperature, magnesium and its alloys are difficult to perform cold working due to the fact plastic deformation of the hexagonal lattice is more complicated than in cubic latticed metals like aluminium, copper and steel. Therefore, magnesium alloys are typically used as cast alloys. Despite the reactive nature of the pure magnesium powder, magnesium metal and its alloys have good resistance to corrosion.
Aluminium is the most common alloying element. Aluminium, zinc, zirconium, and thorium promote precipitation hardening: manganese improves corrosion resistance; and tin improves castability.
We must add, pure magnesium is highly flammable, especially when powdered or shaved into thin strips, though it is difficult to ignite in mass or bulk. It produces intense, bright, white light when it burns. Flame temperatures of magnesium and some magnesium alloys can reach 3,100°C. Burning or molten magnesium reacts violently with water. Once ignited, such fires are difficult to extinguish, because combustion continues in nitrogen (forming magnesium nitride), carbon dioxide (forming magnesium oxide and carbon), and water. Burning magnesium can be quenched by using a Class D dry chemical fire extinguisher. Its flammability is greatly reduced by a small amount of calcium in the alloy.
Thermal Properties of Magnesium Alloys
Thermal properties of materials refer to the response of materials to changes in their temperature and to the application of heat. As a solid absorbs energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Magnesium Alloys
Melting point of Elektron 21 – UNS M12310 is around 550 – 640°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Magnesium Alloys
The thermal conductivity of Elektron 21 – UNS M12310 is 116 W/(m.K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
We hope, this article, Thermal Properties of Magnesium Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.