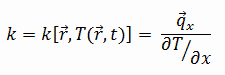Wrought iron is an iron alloy with very low carbon content (less than 0.08%) with respect to cast iron (2.1% to 4%). The microstructure of wrought iron shows dark slag inclusions in ferrite. It is soft, ductile, fibrous variety that is produced from a semifused mass of relatively pure iron globules partially surrounded by slag. It usually contains less than 0.1 percent carbon and 1 or 2 percent slag. Wrought iron is magnetic, corrosion-resistant and easily welded. It has high elasticity and tensile strength. It can be heated and reheated and worked into various shapes. Wrought iron is no longer produced on a commercial scale. The modern functional equivalent of wrought iron is mild steel, also called low-carbon steel. Many products described as wrought iron, such as guard rails, garden furniture and gates, are actually made of mild steel. For example, the Eiffel Tower is a wrought-iron lattice tower.
Thermal Properties of Wrought Iron
Thermal properties of materials refer to the response of materials to changes in their temperature and to the application of heat. As a solid absorbs energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Wrought Iron
Melting point of wrought iron is 1540°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Boiling Point of Wrought Iron
Wrought iron is a multi-element substance, principally of iron, with additions of carbon and impurities. The carbon is mostly in the form of carbides of the alloy metals. The carbides will have higher boiling temperatures than the metal matrix. The boiling point of iron (not wrought iron) is 2860°C, so the boiling point of wrought iron is close to this value.
In general, boiling is a phase change of a substance from the liquid to the gas phase. The boiling point of a substance is the temperature at which this phase change (boiling or vaporization) occurs.
Thermal Conductivity of Wrought Iron
Wrought iron is a multi-element substance, principally of iron, with additions of carbon and impurities. The thermal conductivity of wrought iron is around 60 W/(m.K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
We hope, this article, Thermal Properties of Wrought Iron – Melting Point – Thermal Conductivity, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.