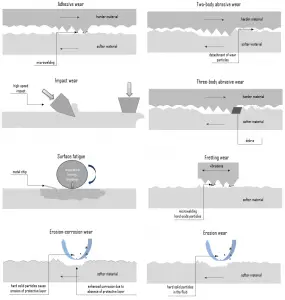
 In general, wear is mechanically induced surface damage that results in the progressive removal of material due to relative motion between that surface and a contacting substance or substances. A contacting substance may consist of another surface, a fluid, or hard, abrasive particles contained in some form of fluid or suspension, such as a lubricant for example. As is with friction, the presence of wear can be either good or bad. Productive, controlled wear can be found in processes like machining, cutting, grinding and polishing. However, in most of the technological applications, the occurrence of wear is highly undesirable and it is an enormously expensive problem since it leads to the deterioration or even failure of components. In terms of safety it is often not as serious (or as sudden) as fracture. This is because wear is usually anticipated.
In general, wear is mechanically induced surface damage that results in the progressive removal of material due to relative motion between that surface and a contacting substance or substances. A contacting substance may consist of another surface, a fluid, or hard, abrasive particles contained in some form of fluid or suspension, such as a lubricant for example. As is with friction, the presence of wear can be either good or bad. Productive, controlled wear can be found in processes like machining, cutting, grinding and polishing. However, in most of the technological applications, the occurrence of wear is highly undesirable and it is an enormously expensive problem since it leads to the deterioration or even failure of components. In terms of safety it is often not as serious (or as sudden) as fracture. This is because wear is usually anticipated.
Certain material characteristics such as hardness, carbide type, and volume percent can have a decided impact on the wear resistance of a material in a given application. Wear, like corrosion, has multiple types and subtypes, is predictable to some extent, and is rather difficult to reliably test and to evaluate in the lab or in service.
Adhesive Wear
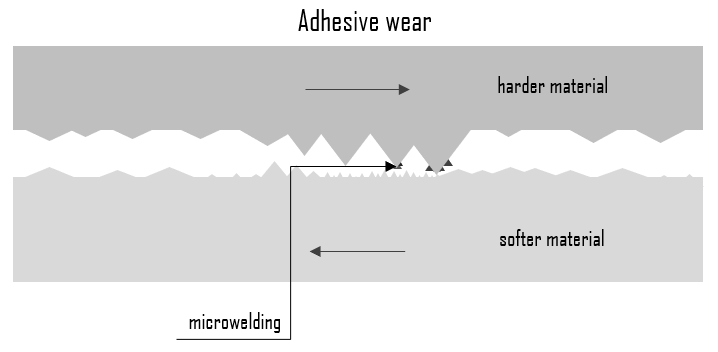 Adhesive wear is originated through the bonding of asperities or microscopic high points (surface roughness) between two sliding materials. When a peak from one surface comes into contact with a peak from the other surface, instantaneous microwelding may take place due to the heat generated by the resulting friction. This results in detachment or material transfer from one surface to the other. For adhesive wear to occur it is necessary for the surfaces to be in intimate contact with each other. This may cause unwanted displacement and attachment of wear debris and material compounds from one surface to another. Adhesive wear can lead to an increase in roughness and the creation of protrusions (i.e., lumps) above the original surface. Surfaces which are held apart by lubricating films, oxide films etc. reduce the tendency for adhesion to occur. In some engineering applications, surfaces slide in air or without lubricant and the resulting wear is termed as dry sliding.
Adhesive wear is originated through the bonding of asperities or microscopic high points (surface roughness) between two sliding materials. When a peak from one surface comes into contact with a peak from the other surface, instantaneous microwelding may take place due to the heat generated by the resulting friction. This results in detachment or material transfer from one surface to the other. For adhesive wear to occur it is necessary for the surfaces to be in intimate contact with each other. This may cause unwanted displacement and attachment of wear debris and material compounds from one surface to another. Adhesive wear can lead to an increase in roughness and the creation of protrusions (i.e., lumps) above the original surface. Surfaces which are held apart by lubricating films, oxide films etc. reduce the tendency for adhesion to occur. In some engineering applications, surfaces slide in air or without lubricant and the resulting wear is termed as dry sliding.
Adhesive wear is dependent on the materials involved, the degree of lubrication provided, and the environment. Adequate lubrication allows smooth, continuous operation of machine elements, reduces the rate of wear, and prevents excessive stresses or seizures at bearings. When lubrication breaks down, components can rub destructively against each other, causing heat, local welding, destructive damage and failure. For instance, austenitic stainless steels (e.g. AISI 304) sliding against themselves are very likely to transfer material and gall, resulting in severe surface damage. Other materials that are prone to adhesive wear include titanium, nickel, and zirconium. On the other hand, aluminium bronze has found increasing recognition for a wide variety of applications requiring resistance to mechanical wear. Its wear-resistance is based on the transfer from the softer metal (aluminium bronze) to the harder metal (steel) and formation of thin layer of softer metal on the harder metal.
For example, the main function of motor oil is to reduce friction and wear on moving parts (to reduce adhesive wear) and to clean the engine from sludge, while a filter is designed to remove contaminants and abrasive particles from engine oil.
Surface Hardness and Wear Resistance
Hardness is important from an engineering standpoint because resistance to wear by either friction or erosion by steam, oil, and water generally increases with hardness. If the hardness of the material is higher than that of the abrasive material, less wear rate will occur.
Case hardening or surface hardening is the process in which hardness the surface (case) of an object is enhanced, while the inner core of the object remains elastic and tough. After this process surface hardness, wear-resistance and fatigue life are enhanced. This is accomplished by several processes such as a carburizing or nitriding process by which a component is exposed to a carbonaceous or nitrogenous atmosphere at elevated temperature. As was written, two main material characteristics are influenced:
- Hardness and wear resistance is significantly enhanced. In materials science, hardness is the ability to withstand surface indentation (localized plastic deformation) and scratching. Hardness is probably the most poorly defined material property because it may indicate resistance to scratching, resistance to abrasion, resistance to indentation or even resistance to shaping or localized plastic deformation. Hardness is important from an engineering standpoint because resistance to wear by either friction or erosion by steam, oil, and water generally increases with hardness.
- Toughness is not negatively influenced. Toughness is the ability of a material to absorb energy and plastically deform without fracturing. One definition of toughness (for high-strain rate, fracture toughness) is that it is a property that is indicative of a material’s resistance to fracture when a crack (or other stress-concentrating defect) is present.
For iron or steel with low carbon content, which has poor to no hardenability of its own, the case-hardening process involves infusing additional carbon or nitrogen into the surface layer. Case hardening is useful in parts such as a cam or ring gear that must have a very hard surface to resist wear, along with a tough interior to resist the impact that occurs during operation. Further, the surface hardening of steel has an advantage over through hardening (that is, hardening the metal uniformly throughout the piece) because less expensive low-carbon and medium-carbon steels can be surface hardened without the problems of distortion and cracking associated with the through hardening of thick sections. A carbon- or nitrogen-rich outer surface layer (or case) is introduced by atomic diffusion from the gaseous phase. The case is normally on the order of 1 mm deep and is harder than the inner core of material.
Typical Wear-resistant Materials
In general, wear is mechanically induced surface damage that results in the progressive removal of material due to relative motion between that surface and a contacting substance or substances. Therefore, there is perfect wear-resistant material and in every case, it depends strongly on many variables (e.g. materials combination, contact pressure, environment, temperature). The hardness of the material is correlated to the wear resistance of the material. If the hardness of the material is less than that of the hardness of the abrasive material, then the wear rate is high. The hardness of material plays a major role in wear resistance. Some materials exhibit special wear characteristics:
- Ni3Al – Alloy. Nickel aluminide is an intermetallic alloy of nickel and aluminium with properties similar to both a ceramic and a metal. Nickel aluminide is unique in that it has very high thermal conductivity combined with high strength at high temperature. These properties, combined with its high strength and low density, make it ideal for special applications like coating blades in gas turbines and jet engines. Composite materials with Ni3Al based alloys as a matrix hardened by, e.g., TiC, ZrO2, WC,SiC and graphene, are advanced materials. In 2005, the most abrasion-resistant material was reportedly created by embedding diamonds in a matrix of nickel aluminide.
- Tungsten Carbide. Impact wear is of the highest impostance in mining nad mineral processing. Mining and mineral processing demand wear-resistant machines and components, because the energies and masses of interacting bodies are significant. For this purposes, materials with the highest wear-resistance must be used. For example, tungsten carbide is used extensively in mining in top hammer rock drill bits, downhole hammers, roller-cutters, long wall plough chisels, long wall shearer picks, raiseboring reamers, and tunnel boring machines.
- Silicon Carbide. Silicon carbide is exceedingly hard, synthetically produced crystalline compound of silicon and carbon. Its chemical formula is SiC. Silicon carbide has a Mohs hardness rating of 9, approaching that of diamond. In addition to hardness, silicon carbide crystals have fracture characteristics that make them extremely useful in grinding wheels. Its high thermal conductivity, together with its high-temperature strength, low thermal expansion, and resistance to chemical reaction, makes silicon carbide valuable in the manufacture of high-temperature applications and other refractories.
- Coated Alloys. Case hardening by surface treatment can be classified further as diffusion treatments or localized heating treatments. Diffusion methods introduce alloying elements that enter the surface by diffusion, either as solid-solution agents or as hardenability agents that assist martensite formation during subsequent quenching. In this process, the concentration of alloying element is increased at the surface of a steel component. Diffusion methods include:
- Carburizing is a case hardening process in which the surface carbon concentration of a ferrous alloy (usually a low-carbon steel) is increased by diffusion from the surrounding environment. Carburizing produces hard, highly wear-resistant surface (medium case depths) of product with excellent capacity for contact load, good bending fatigue strength and good resistance to seizure.
- Nitriding is a case hardening process in which the surface nitrogen concentration of a ferrous is increased by diffusion from the surrounding environment to create case-hardened surface. Nitriding produces hard, highly wear-resistant surface (shallow case depths) of product with fair capacity for contact load, good bending fatigue strength and excellent resistance to seizure.
- Boriding, also called boronizing is a thermochemical diffusion process similar to nitrocarburising in which boron atoms diffuse into the substrate to produce hard and wear-resistant surface layers. The process requires a high treatment temperature (1073-1323 K) and long duration (1-12 h), and can be applied to a wide range of materials such as steels, cast iron, cermets, and non-ferrous alloys.
- Titanium-carbon and Titanium-nitride Hardening. Titanium nitride (an extremely hard ceramic material), or titanium carbide coatings can be used in the tools made of this kind of steels through physical vapor deposition process to improve the performance and life span of the tool. TiN has a Vickers hardness of 1800–2100 and it has metallic gold color.
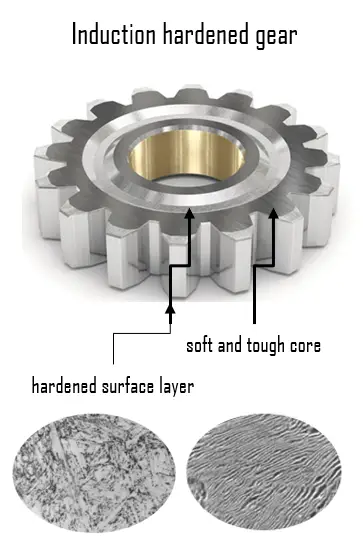 Case Hardened Steels. In order to enhance wear resistance of steels, case hardening based on martensitic transformation is usually performed. Martensitic transformation hardening is one of most common methods of hardening, which is primarily used for steels (i.e. carbon steels as well as stainless steels).
Case Hardened Steels. In order to enhance wear resistance of steels, case hardening based on martensitic transformation is usually performed. Martensitic transformation hardening is one of most common methods of hardening, which is primarily used for steels (i.e. carbon steels as well as stainless steels).- Flame hardening. Flame hardening is a surface hardening technique which uses a single torch with a specially designed head to provide a very rapid means of heating the metal, which is then cooled rapidly, generally using water. This creates a “case” of martensite on the surface, while the inner core of the object remains elastic and tough. It is similar technique as induction hardening. A carbon content of 0.3–0.6 wt% C is needed for this type of hardening.
- Induction hardening. Induction hardening is a surface hardening technique which uses induction coils to provide a very rapid means of heating the metal, which is then cooled rapidly, generally using water. This creates a “case” of martensite on the surface. A carbon content of 0.3–0.6 wt% C is needed for this type of hardening.
- Laser hardening. Laser hardening is a surface hardening technique which uses a laser beam to provide a very rapid means of heating the metal, which is then cooled rapidly (generally by self-quenching). This creates a “case” of martensite on the surface, while the inner core of the object remains elastic and tough.
Some common materials:
-

Nibral Propeller (nickel aluminium bronze) Source: generalpropeller.com Ductile cast iron. Ductile iron, also known as nodular ironor spheroidal graphite iron, is very similar to gray iron in composition, but during solidification the graphite nucleates as spherical particles (nodules) in ductile iron, rather than as flakes. Typical applications for this material include valves, pump bodies, crankshafts, gears, and other automotive and machine components because of its good machinability, fatigue strength, and higher modulus of elasticity (compared to gray iron), and in heavy-duty gears because of its high yield strength and wear resistance.
- Aluminium Bronze. The aluminium bronzes are a family of copper-based alloys offering a combination of mechanical and chemical properties unmatched by any other alloy series. They contain about 5 to 12% of aluminium. Aluminium bronze has found increasing recognition for a wide variety of applications requiring resistance to mechanical wear. Its wear-resistance is based on the transfer from the softer metal (aluminium bronze) to the harder metal (steel) and formation of thin layer of softer metal on the harder metal.
We hope, this article, Adhesive Wear, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.