Tempering
The term tempering refers to a heat treatment which is used to increase the toughness of iron-based alloys. Tempering is usually performed after hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical point for a certain period of time, then allowing it to cool in still air. Tempering makes the metal less hard while making it better able to sustain impacts without breaking. Tempering will cause the dissolved alloying elements to precipitate, or in the case of quenched steels, improve impact strength and ductile properties. Upon heating, the carbon atoms diffuse and react in a series of distinct steps that eventually form Fe3C or an alloy carbide in a ferrite matrix of gradually decreasing stress level.
For tempering, temperature is much more important than time at temperature. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, between 150 and 200 ° and maintain much of the hardness and strength of the quenched martensite and provide a small improvement in ductility and toughness. While springs are tempered at much higher temperatures. Tempering above 425 °C significantly improves ductility and toughness but at the expense of hardness and strength. Under certain conditions, hardness may remain unaffected by tempering or may even be increased as a result of it. Also, those alloy steels that contain one or more of the carbide-forming elements (chromium, molybdenum, vanadium, and tungsten) are capable of secondary hardening: they may become somewhat harder as a result of tempering.
Austempering
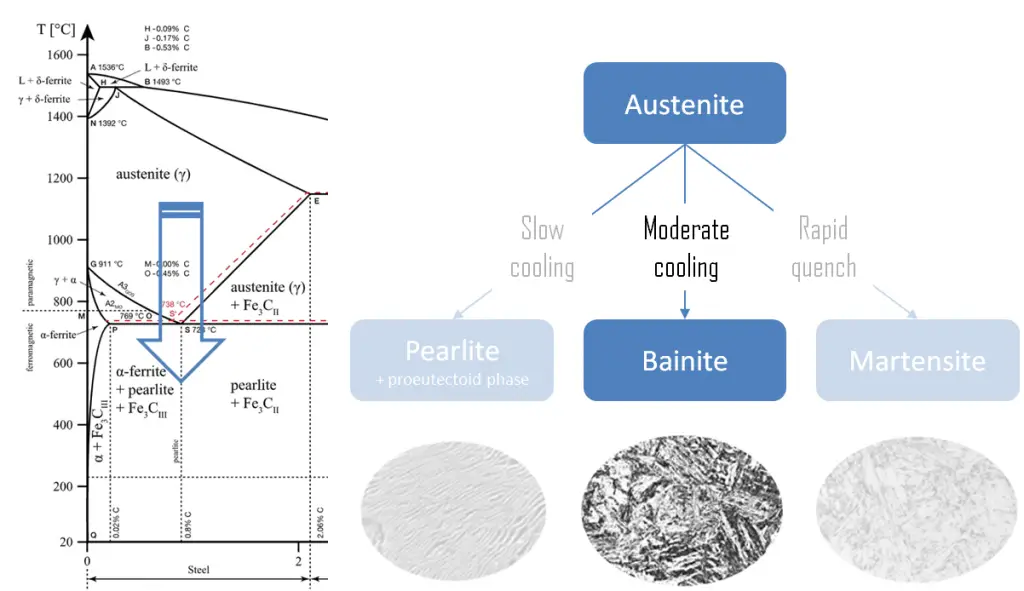 Austempering is a heat treatment used to form pure bainite, a transitional microstructure found between pearlite and martensite. Austempering consists of rapidly cooling the metal part from the austenitizing temperature to about 230 to 400°C, holding at a constant temperature to allow isothermal transformation. To avoid the formation of pearlite or martensite, the steel is quenched in a bath of molten metals or salts. The steel is then held at the bainite-forming temperature, beyond the point where the temperature reaches an equilibrium, until the bainite fully forms. The steel is then removed from the bath and allowed to air-cool, without the formation of either pearlite or martensite. Depending on the holding-temperature, austempering can produce either upper or lower bainite.
Austempering is a heat treatment used to form pure bainite, a transitional microstructure found between pearlite and martensite. Austempering consists of rapidly cooling the metal part from the austenitizing temperature to about 230 to 400°C, holding at a constant temperature to allow isothermal transformation. To avoid the formation of pearlite or martensite, the steel is quenched in a bath of molten metals or salts. The steel is then held at the bainite-forming temperature, beyond the point where the temperature reaches an equilibrium, until the bainite fully forms. The steel is then removed from the bath and allowed to air-cool, without the formation of either pearlite or martensite. Depending on the holding-temperature, austempering can produce either upper or lower bainite.
Bainite is a plate-like microstructure that forms in steels from austenite when cooling rates are not rapid enough to produce martensite but are still fast enough so that carbon does not have enough time to diffuse to form pearlite. The key difference between pearlite and bainite is that the pearlite contains alternating layers of ferrite and cementite whereas the bainite has a plate-like microstructure. A fine non-lamellar structure, bainite commonly consists of cementite and dislocation-rich ferrite. The large density of dislocations in the ferrite present in bainite, and the fine size of the bainite platelets, makes this ferrite harder than it normally would be. Bainitic steels are generally stronger and harder than pearlitic steels; yet they exhibit a superior imact resistance. The hardness of bainite can be between that of pearlite and untempered martensite in the same steel hardness.
Austempering is applicable to most medium-carbon steels and alloy steels. Low-alloy steels are usually restricted to 9.5 mm or thinner sections, while more hardenable steels can be austempered in sections up to 50 mm thick.
The term quenching refers to a heat treatment in which a material is rapidly cooled in water, oil or air to obtain certain material properties, especially hardness. In ferrous alloys, quenching is most commonly used to harden steel by introducing martensite, while non-ferrous alloys will usually become softer than normal. Above this critical temperature, a metal is partially or fully austenitized, the cooling rate of the steel has to be rapid so as to let the austenite transform into metastable bainite or martensite.
The selection of a quenchant medium depends on the hardenability of the particular alloy, the section thickness and shape involved, and the cooling rates needed to achieve the desired micro-structure.
We hope, this article, Austempering, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.