Austenitic stainless steels contain between 16 and 25% of chromium and can also contain nitrogen in solution, both of which contribute to their relatively high corrosion resistance. Austenitic stainless steels are classified with AISI 200- or 300-series designations; the 300-series grades are chromium-nickel alloys, and the 200-series represent a set of compositions in which manganese and/or nitrogen replace some of the nickel. Austenitic stainless steels have the best corrosion resistance of all stainless steels and they have excellent cryogenic properties, and good high-temperature strength. They possess a face-centered cubic (fcc) microstructure that is nonmagnetic, and they can be easily welded. This austenite crystalline structure is achieved by sufficient additions of the austenite stabilizing elements nickel, manganese and nitrogen. Austenitic stainless steel is the largest family of stainless steels, making up about two-thirds of all stainless steel production. Their yield strength is low (200 to 300MPa), which limits their use for structural and other load bearing components. They cannot be hardened by heat treatment but have the useful property of being able to be work hardened to high strength levels whilst retaining a useful level of ductility and toughness. Duplex stainless steels tend to be preferred in such situations because of their high strength and corrosion resistance. The best known grade is AISI 304 stainless, which contains both chromium (between 15% and 20%) and nickel (between 2% and 10.5%) metals as the main non-iron constituents. 304 stainless steel has excellent resistance to a wide range of atmospheric environments and many corrosive media. These alloys are usually characterized as ductile, weldable, and hardenable by cold forming.
Austenitic stainless steels contain between 16 and 25% of chromium and can also contain nitrogen in solution, both of which contribute to their relatively high corrosion resistance. Austenitic stainless steels are classified with AISI 200- or 300-series designations; the 300-series grades are chromium-nickel alloys, and the 200-series represent a set of compositions in which manganese and/or nitrogen replace some of the nickel. Austenitic stainless steels have the best corrosion resistance of all stainless steels and they have excellent cryogenic properties, and good high-temperature strength. They possess a face-centered cubic (fcc) microstructure that is nonmagnetic, and they can be easily welded. This austenite crystalline structure is achieved by sufficient additions of the austenite stabilizing elements nickel, manganese and nitrogen. Austenitic stainless steel is the largest family of stainless steels, making up about two-thirds of all stainless steel production. Their yield strength is low (200 to 300MPa), which limits their use for structural and other load bearing components. They cannot be hardened by heat treatment but have the useful property of being able to be work hardened to high strength levels whilst retaining a useful level of ductility and toughness. Duplex stainless steels tend to be preferred in such situations because of their high strength and corrosion resistance. The best known grade is AISI 304 stainless, which contains both chromium (between 15% and 20%) and nickel (between 2% and 10.5%) metals as the main non-iron constituents. 304 stainless steel has excellent resistance to a wide range of atmospheric environments and many corrosive media. These alloys are usually characterized as ductile, weldable, and hardenable by cold forming.
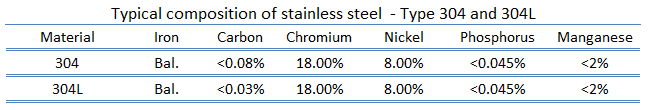
Stainless Steel – Type 304
Type 304 stainless steel (containing 18%-20% chromium and 8%-10.5% nickel) is the most common stainless steel. It is also known as “18/8” stainless steel because of its composition, which includes 18% chromium and 8% nickel. This alloy resists most types of corrosion. It is an austenitic stainless steel and it has also excellent cryogenic properties, and good high-temperature strength as well as good forming and welding properties. It is less electrically and thermally conductive than carbon steel and is essentially non-magnetic.
Type 304L stainless steel, which is widely used in nuclear industry, is an extra-low carbon version of the 304 steel alloy. This grade has slightly lower mechanical properties than the standard 304 grade, but is still widely used thanks to its versatility. The lower carbon content in 304L minimizes deleterious or harmful carbide precipitation as a result of welding. 304L can, therefore, be used “as welded” in severe corrosion environments, and it eliminates the need for annealing. Grade 304 has also good oxidation resistance in intermittent service to 870 °C, and in continuous service to 925 °C.
The body of the reactor vessel is constructed of a high-quality low-alloy carbon steel, and all surfaces that come into contact with reactor coolant are clad with a minimum of about 3 to 10 mm of austenitic stainless steel in order to minimize corrosion. Since grade 304L does not require post-weld annealing, it is extensively used in heavy gauge components.
We hope, this article, Composition of Austenitic Stainless Steel, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.