Zinc is a brittle metal and has a relatively low melting point of 419 °C (787 °F), resists corrosion, is ductile and malleable, and is highly soluble in copper. Zinc and zinc alloys are used in the form of coatings, castings, rolled sheets, drawn wire, forgings, and extrusions. Other uses of zinc are as a major constituent in brassesm nickel-silver alloys, typewriter metal, soft and aluminium solder, and commercial bronze.
Alloys of zinc with small amounts of copper, aluminium, and magnesium are useful in die casting as well as spin casting, especially in the automotive, electrical, and hardware industries. Zinc alloys have low melting points, require relatively low heat input, do not require fluxing or protective atmospheres. Because of their high fluidity, zinc alloys can be cast in much thinner walls than other die castings alloys, and they can be die cast to tighter dimensional tolerances. These zinc alloys are marketed under the name Zamak. The name zamak is an acronym of the German names for the metals of which the alloys are composed: Zink (zinc), Aluminium, Magnesium and Kupfer (copper). The low melting point together with the low viscosity of the alloy makes possible the production of small and intricate shapes.
As a coating, zinc provides corrosion protection for iron and steel (galvanized steel). Coating of steel constitutes the largest single use of zinc, but it is used in large tonnages in zinc alloy castings, as zinc dust and oxide, and in wrought zinc products. Galvanized steel is just plain carbon steel that has been coated with a thin zinc layer. The zinc protects iron by corroding first, but zinc corrodes at much lower rates than do steel. In the event the underlying metal becomes exposed, protection can continue as long as there is zinc close enough to be electrically coupled. After all of the zinc in the immediate area is consumed, localized corrosion of the base metal can occur. Galvanized construction steels are the most common use for galvanized metal, and hundreds of thousands of tons of steel products are galvanized annually worldwide (sheet metal, fences, screen, screws, etc.).
Brass – Copper-Zinc Alloy
Brass is is the generic term for a range of copper-zinc alloys. Brass can be alloyed with zinc in different proportions, which results in a material of varying mechanical, corrosion and thermal properties. Increased amounts of zinc provide the material with improved strength and ductility. Brasses with a copper content greater than 63% are the most ductile of any copper alloy and are shaped by complex cold forming operations. Brass has higher malleability than bronze or zinc. The relatively low melting point of brass and its fluidity make it a relatively easy material to cast. Brass can range in surface color from red to yellow to gold to silver depending on the zinc content. Some of the common uses for brass alloys include costume jewelry, locks, hinges, gears, bearings, hose couplings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins.
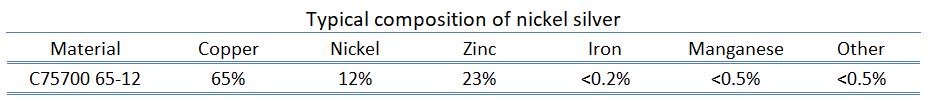
Nickel Silver
Nickel silver, known also as German silver, nickel brass or alpacca, is a copper alloy with nickel and often zinc. The usual formulation is 60% copper, 20% nickel and 20% zinc. For example the alloy C75700 contains 63.5 to 66.5% of Cu, 11.0 to 13.0% of Ni, 0.05% Pb max, 0.25% Fe max, 0.5% Mn max, and balance of Zn. UNS C75700 nickel silver 65-12 copper alloy has good corrosion and tarnish-resistance, and high formability. Nickel silver is named due to its silvery appearance, but it contains no elemental silver unless plated. Nickel silver alloys are used for decorative applications, jewellery, model making, musical instruments (e.g., flutes, clarinets), flutes ball point refills, screws, rivets and fishing rods, test probes.
Zamak – Zamak 3
Zamak is a family of alloys with a base metal of zinc and alloying elements of aluminium, magnesium, and copper. Alloys of zinc with small amounts of copper, aluminium, and magnesium are useful in die casting as well as spin casting, especially in the automotive, electrical, and hardware industries. Zinc alloys have low melting points, require relatively low heat input, do not require fluxing or protective atmospheres. Because of their high fluidity, zinc alloys can be cast in much thinner walls than other die castings alloys, and they can be die cast to tighter dimensional tolerances. These zinc alloys are marketed under the name Zamak. The name zamak is an acronym of the German names for the metals of which the alloys are composed: Zink (zinc), Aluminium, Magnesium and Kupfer (copper). The low melting point together with the low viscosity of the alloy makes possible the production of small and intricate shapes.
For example, Zamak 3 (ASTM AG40A), or Zinc Alloy 3, is the most widely used zinc alloy in the zinc die casting industry and is usually the first choice when considering zinc for die casting for a number of reasons. It provides the best overall combination of strength, castability, dimensional stability, ease of finishing, and cost.
- Excellent physical and mechanical properties
- Excellent castability and long-term dimensional stability
- Excellent finishing characteristics for plating, painting, and chromate treatments
- Excellent damping capacity and vibration attenuation in comparison to aluminum die cast alloys
Typical applications include die castings such as automotive parts, household appliances and fixtures, office and computer equipment, building hardware.
We hope, this article, Composition of Zinc Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.

