Corrosion is the deterioration of a material due to chemical interaction with its environment. It is natural process in which metals convert its structure into a more chemically-stable form such as oxides, hydroxides, or sulfides. The consequences of corrosion are all too common. Familiar examples include the rusting of automotive body panels and pipings and many tools. Corrosion is usually a negative phenomenon, since it is associated with mechanical failure of an object. Metal atoms are removed from a structural element until it fails, or oxides build up inside a pipe until it is plugged. All metals and alloys are subject to corrosion. Even the noble metals, such as gold, are subject to corrosive attack in some environments.
Crevice Corrosion
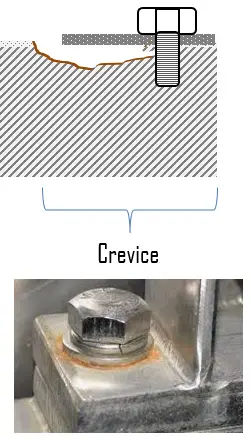 Crevice corrosion refers to the localized corrosion that occurs at the crevice or gap between two or more joining metals. Crevice corrosion is a type of pitting corrosion that occurs specifically within the low flow region of a crevice. This type of attack is usually associated with small volumes of stagnant solution caused by holes, gaskets surface, lap joints, surface deposits, and crevice under bolt and rivets heads. The damage takes place due to the difference in constituents’ concentration, mainly oxygen, in the surfaces involved. Crevice corrosion can progress very rapidly (tens to hundreds of times faster than the normal rate of general corrosion in the same given solution).
Crevice corrosion refers to the localized corrosion that occurs at the crevice or gap between two or more joining metals. Crevice corrosion is a type of pitting corrosion that occurs specifically within the low flow region of a crevice. This type of attack is usually associated with small volumes of stagnant solution caused by holes, gaskets surface, lap joints, surface deposits, and crevice under bolt and rivets heads. The damage takes place due to the difference in constituents’ concentration, mainly oxygen, in the surfaces involved. Crevice corrosion can progress very rapidly (tens to hundreds of times faster than the normal rate of general corrosion in the same given solution).
Crevice corrosion is a hazard due to the possible rapid penetration of the metal with little overall loss of mass. Crevice corrosion is minimized by:
- Crevice corrosion may be prevented by using welded instead of riveted or bolted joints
- Using the correct metals and alloys that are less susceptible to the corrosion
- Avoiding agents in the medium that cause pitting
- Designing the system and components such that no crevices are present
We hope, this article, Crevice Corrosion, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.