Line defects are generally many atoms in length. Line defects are called dislocations and occur in crystalline materials only. Dislocations are especially important in materials science, because they help determine the mechanical strength of materials. There are two basic types of dislocations, the edge dislocation and the screw dislocation. Mixed dislocations, combining aspects of both types, are also common. It is important to note that dislocations cannot end inside a crystal. They must end at a crystal edge or other dislocation, or they must close back on themselves.
Early materials studies led to the computation of the theoretical strengths of perfect crystals. But these theoretical strengths were many times greater than those actually measured. During the 1930s it was theorized that this discrepancy in mechanical strengths could be explained by a type of linear crystalline defect that has come to be known as a dislocation. The term ‘dislocation’ referring to a defect on the atomic scale was coined by G. I. Taylor in 1934.
Edge Dislocation
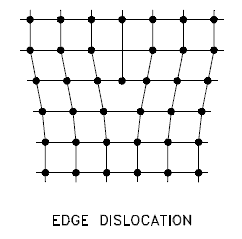
Edge dislocation centers around the edge dislocation line that is defined along the end of the extra half-plane of atoms. The imperfection may extend in a straight line all the way through the crystal or it may follow an irregular path. It may also be short, extending only a small distance into the crystal causing a slip of one atomic distance along the glide plane (direction the edge imperfection is moving). Macroscopic plastic deformation simply corresponds to permanent deformation that results from the movement of dislocations, or slip, in response to an applied shear stress. Dislocations can move if the atoms from one of the surrounding planes break their bonds and rebond with the atoms at the terminating edge. Understanding the movement of a dislocation is key to understanding why dislocations allow deformation to occur at much lower stress than in a perfect crystal. Dislocation motion is analogous to movement of a caterpillar. The caterpillar would have to exert a large force to move its entire body at once. Instead it moves the rear portion of its body forward a small amount and creates a hump. The hump then moves forward and eventual moves all of the body forward by a small amount. The slip occurs when the crystal is subjected to a stress, and the dislocation moves through the crystal until it reaches the edge or is arrested by another dislocation.
We hope, this article, Edge Dislocation – Crystallographic Defects, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.