Ferrite or α-ferrite is a body-centered cubic structure phase of iron which exists below temperatures of 912°C for low concentrations of carbon in iron. α-ferrite can only dissolve up to 0.02 percent of carbon at 727°C. This is because of the configuration of the iron lattice which forms a BCC crystal structure. In a bcc (BCC) arrangement of atoms, the unit cell consists of eight atoms at the corners of a cube and one atom at the body center of the cube. In a bcc arrangement, a unit cell contains (8 corner atoms × ⅛) + (1 center atom × 1) = 2 atoms. The packing is more efficient (68%) than simple cubic and the structure is a common one for alkali metals and early transition metals.
The primary phase of low-carbon or mild steel and most cast irons at room temperature is ferromagnetic α-Fe. It has a hardness of approximately 80 Brinell. Mild steel (carbon steel with up to about 0.2 wt% C) consist mostly of α-Fe and increasing amounts of cementite (Fe3C, an iron carbide). The mixture adopts a laminar structure called pearlite. Since bainite and pearlite each contain α-Fe as a component, any iron-carbon alloy will contain some amount of α-Fe if it is allowed to reach equilibrium at room temperature. The amount of α-Fe depends on the cooling process.
δ-ferrite
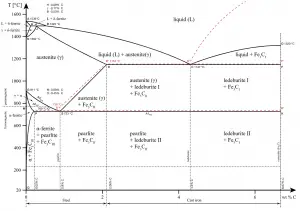
δ-ferrite phase has a similar structure, a body-centered cubic (BCC) crystal structure, as that of α-ferrite but exists only at high temperatures. The phase can be spotted at the top left corner in the graph. It has a melting point of 1538°C.
Low-Carbon Steel
Low-carbon steel, also known as mild steel is now the most common form of steel because its price is relatively low while it provides material properties that are acceptable for many applications. Low-carbon steel contains approximately 0.05–0.25% carbon making it malleable and ductile. Mild steel has a relatively low tensile strength, but it is cheap and easy to form; surface hardness can be increased through carburizing.

Typical applications include automobile body components, structural shapes (e.g., I-beams, channel and angle iron), and sheets that are used in pipelines, buildings. For example, A36 steel is a common structural steel in the United States. Low-carbon steel sheets used for car body applications, for example, are subjected to a variety of forming operations, including deep drawing. Microstructures consist of ferrite and pearlite constituents. As a consequence, these alloys are relatively soft and weak but have outstanding ductility and toughness. In addition, they are machinable, weldable, and, of all steels, are the least expensive to produce. Density of this metal is 7861.093 kg/m³ (0.284 lb/in³) and the tensile strength is a maximum of 500 MPa (72500 psi).
Ferritic Stainless Steel
In ferritic stainless steels, carbon is kept to low levels (C<0.08%) and the chromium content can range from 10.50 to 30.00%. They are called ferritic alloys because they contain primarily ferritic microstructures at all temperatures and cannot be hardened through heat treating and quenching. They are classified with AISI 400-series designations. While some ferritic grades contain molybdenum (up to 4.00%), only chromium is present as the main metallic alloying element. They are usually limited in use to relatively thin sections due to lack of toughness in welds. Moreover, they have relatively poor high-temperature strength. Ferritic steels are chosen for their resistance to stress corrosion cracking, which makes them an attractive alternative to austenitic stainless steels in applications where chloride-induced SCC is prevalent.
Other Common Phases in Steels and Irons
Heat treatment of steels requires an understanding of both the equilibrium phases and the metastable phases that occur during heating and/or cooling. For steels, the stable equilibrium phases include:
- Ferrite. Ferrite or α-ferrite is a body-centered cubic structure phase of iron which exists below temperatures of 912°C for low concentrations of carbon in iron. α-ferrite can only dissolve up to 0.02 percent of carbon at 727°C. This is because of the configuration of the iron lattice which forms a BCC crystal structure. The primary phase of low-carbon or mild steel and most cast irons at room temperature is ferromagnetic α-Fe.
- Austenite. Austenite, also known as gamma-phase iron (γ-Fe), is a non-magnetic face-centered cubic structure phase of iron. Austenite in iron-carbon alloys is generally only present above the critical eutectoid temperature (723°C), and below 1500°C, depending on carbon content. However, it can be retained to room temperature by alloy additions such as nickel or manganese. Carbon plays an important role in heat treatment, because it expands the temperature range of austenite stability. Higher carbon content lowers the temperature needed to austenitize steel—such that iron atoms rearrange themselves to form an fcc lattice structure. Austenite is present in the most commonly used type of stainless steel, which are very well known for their corrosion resistance.
- Graphite. Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater strength.
- Cementite. Cementite (Fe3C) is a metastable compound, and under some circumstances it can be made to dissociate or decompose to form α-ferrite and graphite, according to the reaction: Fe3C → 3Fe (α) + C (graphite). Cementite in its pure form is a ceramic and it is hard and brittle which makes it suitable for strengthening steels. Its mechanical properties are a function of its microstructure, which depends upon how it is mixed with ferrite.
The metastable phases are:
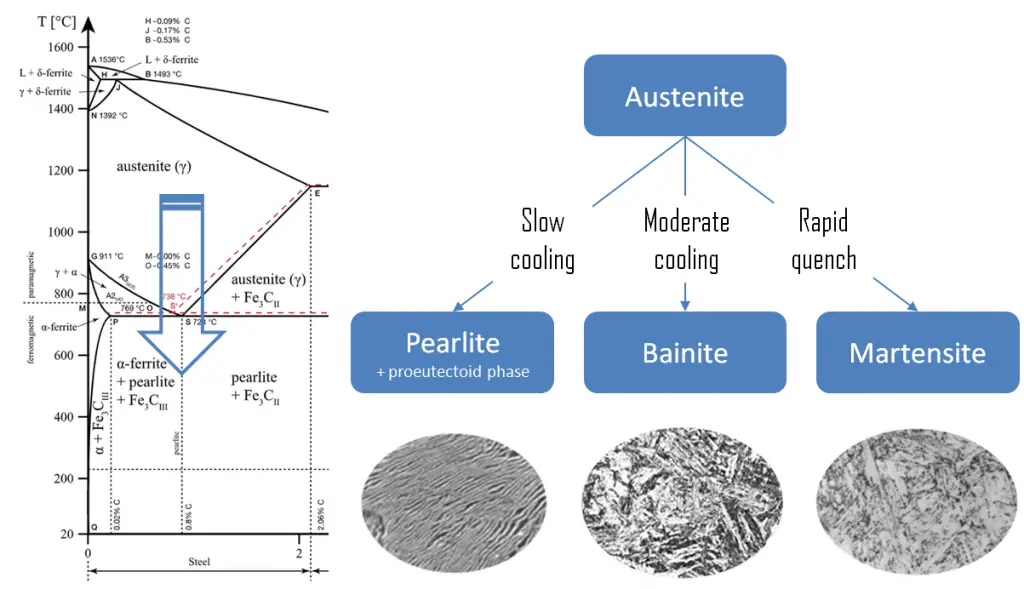 Pearlite. In metallurgy, pearlite is a layered metallic structure of two-phases, which compose of alternating layers of ferrite (87.5 wt%) and cementite (12.5 wt%) that occurs in some steels and cast irons. It is named for its resemblance to mother of pearl.
Pearlite. In metallurgy, pearlite is a layered metallic structure of two-phases, which compose of alternating layers of ferrite (87.5 wt%) and cementite (12.5 wt%) that occurs in some steels and cast irons. It is named for its resemblance to mother of pearl.- Martensite. Martensite is a very hard metastable structure with a body-centered tetragonal (BCT) crystal structure. Martensite is formed in steels when the cooling rate from austenite is at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite (Fe3C).
- Bainite. Bainite is a plate-like microstructure that forms in steels from austenite when cooling rates are not rapid
enough to produce martensite but are still fast enough so that carbon does not have enough time to diffuse to form pearlite. Bainitic steels are generally stronger and harder than pearlitic steels; yet they exhibit a desirable combination of strength and ductility.
We hope, this article, Ferrite, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.