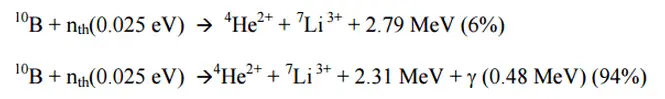Isotope
 In nuclear physics and nuclear chemistry, the various species of atoms whose nuclei contain particular numbers of protons and neutrons are called nuclides. Nuclides are also characterized by its nuclear energy states (e.g. metastable nuclide 242mAm). Each nuclide is denoted by chemical symbol of the element (this specifies Z) with the atomic mass number as superscript. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Hydrogen (H), for example , consist of one electron and one proton. The number of neutrons in a nucleus is known as the neutron number and is given the symbol N. The total number of nucleons, that is, protons and neutrons in a nucleus, is equal to Z + N = A, where A is called the atomic mass number.
In nuclear physics and nuclear chemistry, the various species of atoms whose nuclei contain particular numbers of protons and neutrons are called nuclides. Nuclides are also characterized by its nuclear energy states (e.g. metastable nuclide 242mAm). Each nuclide is denoted by chemical symbol of the element (this specifies Z) with the atomic mass number as superscript. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Hydrogen (H), for example , consist of one electron and one proton. The number of neutrons in a nucleus is known as the neutron number and is given the symbol N. The total number of nucleons, that is, protons and neutrons in a nucleus, is equal to Z + N = A, where A is called the atomic mass number.
Thus the symbol 1H refers to the nuclide of hydrogen with a single proton as nucleus. 2H is the hydrogen nuclide with a neutron as well as a proton in the nucleus (2H is also called deuterium or heavy hydrogen). Atoms such as 1H, 2H whose nuclei contain the same number of protons but different number of neutrons (different A) are isotopes. Uranium, for instance, has three isotopes occurring in nature – 238U, 235U and 234U. All have 92 protons in their nuclei. But, whereas the 235U isotope has 143 neutrons in its nucleus, that of the 238U isotope contains 146 neutrons. The stable isotopes (plus a few of the unstable isotopes) are the atoms that are found in the naturally occurring elements in nature. However, they are not found in equal amounts. Some isotopes of a given element are more abundant than others. For example 99,27% of naturally occuring uranium atoms are the isotope 238U, 0,72% are the isotope 235U and 0,0055% are the isotope 234U.
Because all the isotopes of a given element exhibit the same chemical properties, their separation is very difficult by chemical means. The traditional methods of isotope separation are based directly on the atomic weight of the isotope or based on the small differences in chemical reaction rates produced by different atomic weights. These methods have included mass spectometry, centrifugal separation and processes that depend on the different rates at which heavy and light particles diffuse.
Isotopes of the highest importance in reactor physics
Uranium
Uranium is a naturally-occurring chemical element with atomic number 92 which means there are 92 protons and 92 electrons in the atomic structure. The chemical symbol for uranium is U. Uranium is commonly found at low levels (a few ppm – parts per million) in all rocks, soil, water, plants, and animals (including humans). Uranium occurs also in seawater, and can be recovered from the ocean water. Significant concentrations of uranium occur in some substances such as uraninite (the most common uranium ore), phosphate rock deposits, and other minerals.
Natural uranium consists primarily of isotope 238U (99.28%), therefore the atomic mass of uranium element is close to the atomic mass of 238U isotope (238.03u). Natural uranium also consists of two other isotopes: 235U (0.71%) and 234U (0.0054%). The abundance of isotopes in the nature is caused by difference in the half-lifes. All three naturally-occurring isotopes of uranium (238U, 235U and 234U) are unstable. On the other hand these isotopes (except 234U) belong to primordial nuclides, because their half-life is comparable to the age of the Earth (~4.5×109 years for 238U).
In nuclear reactors we have to consider three artificial isotopes, 236U, 233U and 232U. These are produced by transmutation in nuclear reactors from 235U and 232Th.
Xenon
Xenon is a naturally-occurring chemical element with atomic number 54 which means there are 54 protons and 54 electrons in the atomic structure. The chemical symbol for xenon is Xe. Xenon is a colorless, dense, odorless noble gas found in the Earth’s atmosphere in trace amounts.
Natural xenon consists of eight stable isotopes, 124Xe (0.095%), 126Xe (0.089%), 128Xe (1.91%), 129Xe (26.4%), 130Xe (4.07%), 131Xe (21.23%), 132Xe (26.91%), 134Xe (10.44%), and one isotope with very long half-life 136Xe (8.86%).
In nuclear industry, especially artificial xenon 135 has a tremendous impact on the operation of a nuclear reactor. For physicists and for reactor operators, it is important to understand the mechanisms that produce and remove xenon from the reactor to predict how the reactor will respond following changes in power level.
Another important isotope is the xenon 133, which has half-life of 5.2 days, and its presence in a reactor coolant indicates (together with xenon 135) a possible failure of fuel cladding. A new defect will often result in a step increase in only the Xe-133 activity, which is measured from reactor coolant. As the defect enlarges, the release rate of the soluble, longer-lived nuclides, particularly I-131, I-134, Cs-134, and Cs-137 will increase.
Boron
Boron is a naturally-occurring chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure. The chemical symbol for boron is B.
Natural boron consists primarily of two stable isotopes, 11B (80.1%) and 10B (19.9%). In nuclear industry boron is commonly used as a neutron absorberdue to the high neutron cross-section of isotope 10B. Its (n,alpha) reaction cross-section for thermal neutrons is about 3840 barns (for 0.025 eV neutron). Isotope 11B has absorption cross-section for thermal neutrons about 0.005 barns (for 0.025 eV neutron). Most of (n,alpha) reactions of thermal neutrons are 10B(n,alpha)7Li reactions accompanied by 0.48 MeV gamma emission.
Moreover, isotope 10B has high (n,alpha) reaction cross-section along the entire neutron energy spectrum. The cross-sections of most other elements becomes very small at high energies as in the case of cadmium. The cross-section of 10B decreases monotonically with energy. For fast neutrons its cross-section is on the order of barns.
We hope, this article, Isotope – Nuclide, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.