Corrosion is the deterioration of a material due to chemical interaction with its environment. It is natural process in which metals convert its structure into a more chemically-stable form such as oxides, hydroxides, or sulfides. The consequences of corrosion are all too common. Familiar examples include the rusting of automotive body panels and pipings and many tools. Corrosion is usually a negative phenomenon, since it is associated with mechanical failure of an object. Metal atoms are removed from a structural element until it fails, or oxides build up inside a pipe until it is plugged. All metals and alloys are subject to corrosion. Even the noble metals, such as gold, are subject to corrosive attack in some environments.
Pitting Corrosion
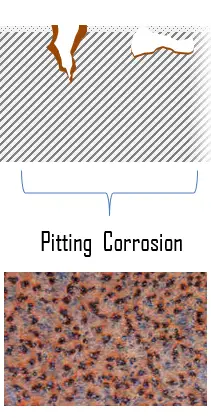 Pitting corrosion, characterized by sharply defined holes, is one of the most insidious forms of corrosion. They ordinarily penetrate from the top of a horizontal surface downward in a nearly vertical direction. It is supposed that gravity causes the pits to grow downward. Pitting corrosion can cause failure by perforation while producing only a small weight loss on the metal. This perforation can be difficult to detect and its growth rapid, leading to unexpected loss of function of the component. Pitting corrosion has also been associated with both crevice and galvanic corrosion. Metal deposition (copper ions plated on a steel surface) can also create sites for pitting attack.
Pitting corrosion, characterized by sharply defined holes, is one of the most insidious forms of corrosion. They ordinarily penetrate from the top of a horizontal surface downward in a nearly vertical direction. It is supposed that gravity causes the pits to grow downward. Pitting corrosion can cause failure by perforation while producing only a small weight loss on the metal. This perforation can be difficult to detect and its growth rapid, leading to unexpected loss of function of the component. Pitting corrosion has also been associated with both crevice and galvanic corrosion. Metal deposition (copper ions plated on a steel surface) can also create sites for pitting attack.
Causes of pitting corrosion include:
- Local inhomogeneity on the metal surface
- Local loss of passivity
- Mechanical or chemical rupture of a protective oxide coating
- Galvanic corrosion from a relatively distant cathode
With corrosion-resistant alloys, such as stainless steels, the most common cause of pitting corrosion is highly localized destruction of passivity by contact with moisture that contains halide ions, particularly chlorides. However, alloying with about 2% molybdenum enhances their resistance significantly. Chloride-induced pitting of stainless steels usually results in undercutting, producing enlarged subsurface cavities or caverns.
Pitting Corrosion – Protection
Pitting corrosion is a hazard due to the possible rapid penetration of the metal with little overall loss of mass. Pitting corrosion is minimized by:
- Avoiding stagnant conditions
- Using the correct metals and alloys that are less susceptible to the corrosion
- Avoiding agents in the medium that cause pitting
- Designing the system and components such that no crevices are present
We hope, this article, Pitting Corrosion, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.