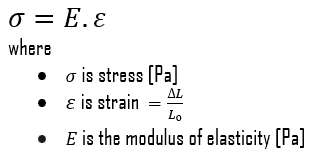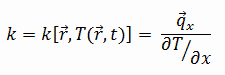Aluminium alloys are based on aluminium, in which the main alloying elements are Cu, Mn, Si, Mg, Mg+Si, Zn. Aluminium alloy compositions are registered with The Aluminum Association. The aluminium alloys are divided into 9 families (Al1xxx to Al9xxx). The different families of alloys and the major alloying elements are:
- 1xxx: no alloying elements
- 2xxx: Copper
- 3xxx: Manganese
- 4xxx: Silicon
- 5xxx: Magnesium
- 6xxx: Magnesium and silicon
- 7xxx: Zinc, magnesium, and copper
- 8xxx: other elements which are not covered by other series
There are also two principal classifications, namely casting alloys and wrought alloys, both of which are further subdivided into the categories heat-treatable and non-heat-treatable. Aluminium alloys containing alloying elements with limited solid solubility at room temperature and with a strong temperature dependence of solid solubility (for example Cu) can be strengthened by a suitable thermal treatment (precipitation hardening). The strength of heat treated commercial Al alloys exceeds 550 MPa.
Aluminium Alloys – Series 2000 – Duralumin
Aluminium alloys of 2000 series are alloyed with copper, they can be precipitation hardened to strengths comparable to steel. Formerly referred to as duralumin, they were once the most common aerospace alloys, but were susceptible to stress corrosion cracking and are increasingly replaced by 7000 series in new designs. In addition to aluminium, the main materials in duralumin are copper, manganese and magnesium.
Duralumin (also called duraluminum, duraluminium, duralum, dural(l)ium, or dural) is a strong, lightweight alloy of aluminium discovered in 1910 by Alfred Wilm, a German metallurgist. He discovered that after quenching, an aluminium alloy containing 4% copper would slowly harden when left at room temperature for several days. This process is now known as natural aging. He also designed an alloy (Duralumin) suitable for strengthening by this process in what is now known as preciptitation hardening. Although an explanation for the phenomenon was not provided until 1919, duralumin was one of the first “age hardening” alloys used.
Summary
| Name | Duralumin |
| Phase at STP | N/A |
| Density | 2780 kg/m3 |
| Ultimate Tensile Strength | 450 MPa |
| Yield Strength | 300 MPa |
| Young’s Modulus of Elasticity | 76 GPa |
| Brinell Hardness | 120 BHN |
| Melting Point | 570 °C |
| Thermal Conductivity | 140 W/mK |
| Heat Capacity | 900 J/g K |
| Price | 6 $/kg |
In terms of age hardening, solution annealed aluminium-copper alloys can be aged naturally at room temperature for four days or more to obtain maximum properties such as hardness and strength. This process is known as natural aging. At room temperature, the solubility of copper in aluminium drops to a small fraction of 1%. At this point, the copper solute is locked inside the aluminium lattice (matrix), but must “precipitate” out of the supersaturated aluminium lattice. The aging process also can be accelerated to a matter of hours after solution treatment and quenching by heating the supersaturated alloy to a specific temperature and holding at that temperature for a specified time. This process is called artificial aging.
Duralumin is relatively soft, ductile and easily workable under normal temperature. The alloy can be rolled, forged and extruded into various forms and products. The light weight and high strength of duralumin when compared to steel enabled its application in aircraft construction. Although the addition of copper improves strength, it also makes these alloys susceptible to corrosion. The electrical and heat conductivity of duralumin is less than that of pure aluminium and more than that of steel.
Strength of Aluminium Alloys – Duralumin
In mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. Strength of materials basically considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. Strength of a material is its ability to withstand this applied load without failure or plastic deformation.
Ultimate Tensile Strength – Duralumin
Ultimate tensile strength of 2024 aluminium alloy depends greatly on the temper of the material, but it is about 450 MPa.
 The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after the ultimate strength has been achieved. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for an aluminum to as high as 3000 MPa for very high-strength steels.
The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after the ultimate strength has been achieved. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for an aluminum to as high as 3000 MPa for very high-strength steels.
Yield Strength
Yield strength of 2024 aluminium alloy depends greatly on the temper of the material, but it is about 300 MPa.
The yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning plastic behavior. Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically whereas yield point is the point where nonlinear (elastic + plastic) deformation begins. Prior to the yield point, the material will deform elastically and will return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible. Some steels and other materials exhibit a behaviour termed a yield point phenomenon. Yield strengths vary from 35 MPa for a low-strength aluminum to greater than 1400 MPa for very high-strength steels.
Young’s Modulus of Elasticity
Young’s modulus of elasticity of 2024 aluminium alloy is about 76 GPa.
The Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests. Up to a limiting stress, a body will be able to recover its dimensions on removal of the load. The applied stresses cause the atoms in a crystal to move from their equilibrium position. All the atoms are displaced the same amount and still maintain their relative geometry. When the stresses are removed, all the atoms return to their original positions and no permanent deformation occurs. According to the Hooke’s law, the stress is proportional to the strain (in the elastic region), and the slope is Young’s modulus. Young’s modulus is equal to the longitudinal stress divided by the strain.
Hardness of Aluminium Alloys – Duralumin
Brinell hardness of 2024 aluminium alloy depends greatly on the temper of the material, but it is approximately 110 MPa.
Rockwell hardness test is one of the most common indentation hardness tests, that has been developed for hardness testing. In contrast to Brinell test, the Rockwell tester measures the depth of penetration of an indenter under a large load (major load) compared to the penetration made by a preload (minor load). The minor load establishes the zero position. The major load is applied, then removed while still maintaining the minor load. The difference between depth of penetration before and after application of the major load is used to calculate the Rockwell hardness number. That is, the penetration depth and hardness are inversely proportional. The chief advantage of Rockwell hardness is its ability to display hardness values directly. The result is a dimensionless number noted as HRA, HRB, HRC, etc., where the last letter is the respective Rockwell scale.
The Rockwell C test is performed with a Brale penetrator (120°diamond cone) and a major load of 150kg.
Thermal Properties of Aluminium Alloys – Duralumin
Thermal properties of materials refer to the response of materials to changes in their thermodynamics/thermodynamic-properties/what-is-temperature-physics/”>temperature and to the application of heat. As a solid absorbs thermodynamics/what-is-energy-physics/”>energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Aluminium Alloys
Melting point of 2024 aluminium alloy is around 570°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Aluminium Alloys
The thermal conductivity of 2024 aluminium alloy is 140 W/(m.K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
U.S. Department of Energy, Material Science. DOE Fundamentals Handbook, Volume 1 and 2. January 1993.
U.S. Department of Energy, Material Science. DOE Fundamentals Handbook, Volume 2 and 2. January 1993.
William D. Callister, David G. Rethwisch. Materials Science and Engineering: An Introduction 9th Edition, Wiley; 9 edition (December 4, 2013), ISBN-13: 978-1118324578.
Eberhart, Mark (2003). Why Things Break: Understanding the World by the Way It Comes Apart. Harmony. ISBN 978-1-4000-4760-4.
Gaskell, David R. (1995). Introduction to the thermodynamics of Materials (4th ed.). Taylor and Francis Publishing. ISBN 978-1-56032-992-3.
González-Viñas, W. & Mancini, H.L. (2004). An Introduction to Materials Science. Princeton University Press. ISBN 978-0-691-07097-1.
Ashby, Michael; Hugh Shercliff; David Cebon (2007). Materials: engineering, science, processing and design (1st ed.). Butterworth-Heinemann. ISBN 978-0-7506-8391-3.
J. R. Lamarsh, A. J. Baratta, Introduction to Nuclear Engineering, 3d ed., Prentice-Hall, 2001, ISBN: 0-201-82498-1.
We hope, this article, Series 2000 – Duralumin, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.