This article contains comparison of key thermal and atomic properties of cobalt and tungsten, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Cobalt vs Tungsten.
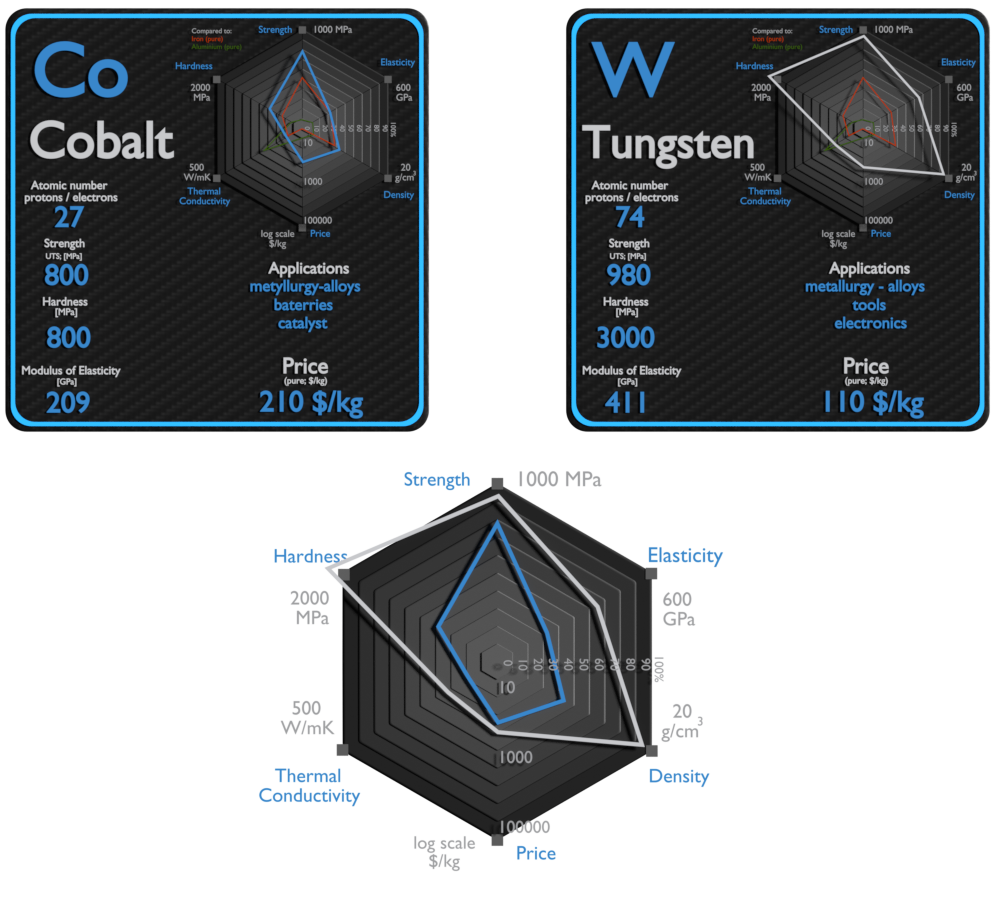
Cobalt and Tungsten – About Elements


Source: www.luciteria.com
Cobalt and Tungsten – Applications
Cobalt
Cobalt has been used in many industrial, commercial, and military applications. Cobalt is primarily used in lithium-ion batteries, and in the manufacture of magnetic, wear-resistant and high-strength alloys. Cobalt-based Superalloys. This class of alloys is relatively new. In 2006, Sato et al. discovered a new phase in the Co–Al–W system. Unlike other superalloys, cobalt-base alloys are characterized by a solid-solution-strengthened austenitic (fcc) matrix in which a small quantity of carbide is distributed. While not used commercially to the extent of Ni-based superalloys, alloying elements found in research Co-based alloys are C, Cr, W, Ni, Ti, Al, Ir, and Ta. They possess better weldability and thermal fatigue resistance as compared to nickel based alloy. Moreover, they have excellent corrosion resistance at high temperatures (980-1100 °C) because of their higher chromium contents. Several cobalt compounds are oxidation catalysts. Typical catalysts are the cobalt carboxylates (known as cobalt soaps). They are also used in paints, varnishes, and inks as “drying agents” through the oxidation of drying oils.
Tungsten
Tungsten is widely used metal. Approximately half of the tungsten is consumed for the production of hard materials – namely tungsten carbide – with the remaining major use being in alloys and steels. Mining and mineral processing demand wear-resistant machines and components, because the energies and masses of interacting bodies are significant. For this purposes, materials with the highest wear-resistance must be used. For example, tungsten carbide is used extensively in mining in top hammer rock drill bits, downhole hammers, roller-cutters, long wall plough chisels, long wall shearer picks, raiseboring reamers, and tunnel boring machines. The remaining 40% is generally used to make various alloys and specialty steels, electrodes, filaments, wires, as well as diverse components for electric, electronic, heating, lighting, and welding applications. High-speed steels are complex iron-base alloys of carbon, chromium, vanadium, molybdenum, or tungsten (as much as 18%), or combinations there of.
Cobalt and Tungsten – Comparison in Table
| Element | Cobalt | Tungsten |
| Density | 8.9 g/cm3 | 19.25 g/cm3 |
| Ultimate Tensile Strength | 800 MPa | 980 MPa |
| Yield Strength | 220 MPa | 750 MPa |
| Young’s Modulus of Elasticity | 209 GPa | 411 GPa |
| Mohs Scale | 5 | 7.5 |
| Brinell Hardness | 800 MPa | 3000 MPa |
| Vickers Hardness | 1040 MPa | 3500 MPa |
| Melting Point | 1495 °C | 3410 °C |
| Boiling Point | 2927 °C | 59300 °C |
| Thermal Conductivity | 100 W/mK | 170 W/mK |
| Thermal Expansion Coefficient | 13 µm/mK | 4.5 µm/mK |
| Specific Heat | 0.42 J/g K | 0.13 J/g K |
| Heat of Fusion | 16.19 kJ/mol | 35.4 kJ/mol |
| Heat of Vaporization | 376.5 kJ/mol | 824 kJ/mol |