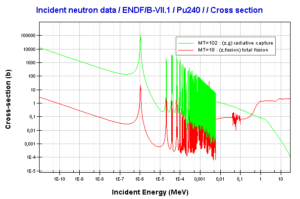
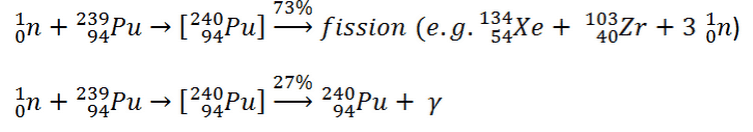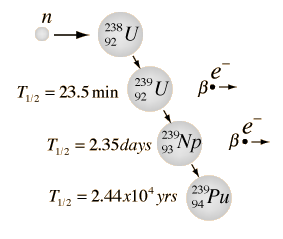What is Plutonium
Plutonium is a transuranic chemical element with atomic number 94 which means there are 94 protons and 94 electrons in the atomic structure. The chemical symbol for plutonium is Pu. It is a man-made isotope and is created from uranium in nuclear reactors. Therefore we can find this element in irradiated nuclear fuel or as a fissile material in nuclear weapons. In fact, scientists have found trace amounts of naturally-occurring plutonium. Four isotopes (238Pu, 239Pu, 240Pu and 244Pu) can be found in the nature.
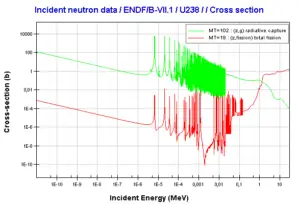
- Trace amounts of 239Pu originate in radiative capture of neutron on 238U. Free neutrons come from a spontaneous fission reaction of 238U.
- Trace amounts of 244Pu originate in its relatively long half-life of about 80 million years.
- The isotope 240Pu is in a decay chain of the isotope 244Pu. These nuclei are present in the unalterable proportions of the radioactive equilibrium between 244Pu and 240Pu.
- Extremely small amounts of 238Pu originate in extremely rare double negative beta decay of naturally-occurring isotope 238U.
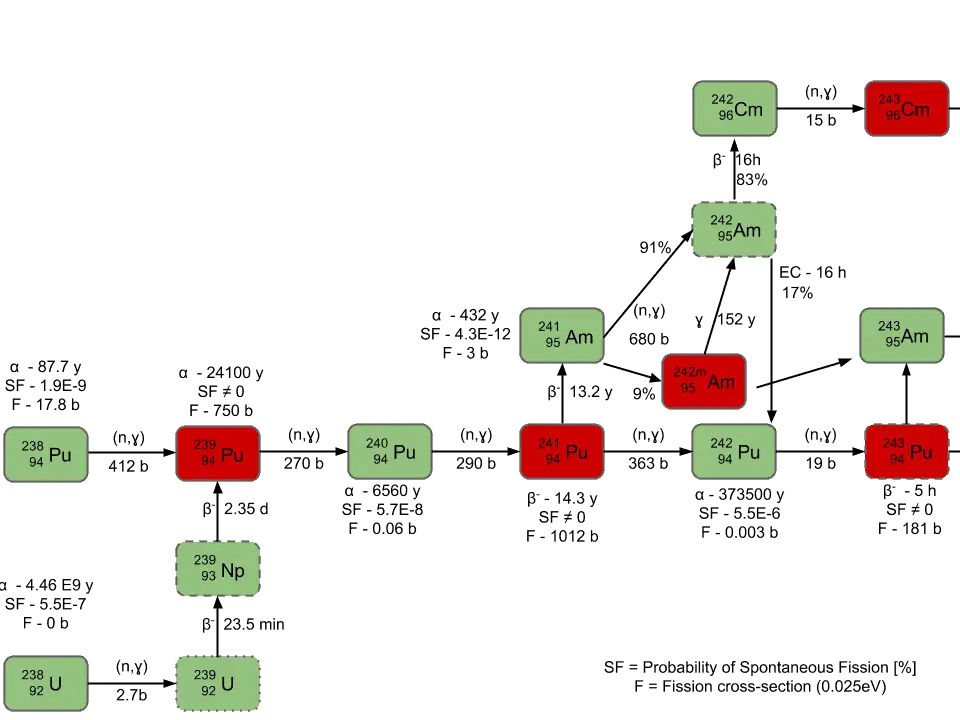
Neutron capture may also be used to create fissile 239Pu from 238U, which is the dominant constituent of naturally occurring uranium (99.28%). Absorption of a neutron in the 238U nucleus yields 239U. The half-life of 239U is approximately 23.5 minutes. 239U decays (negative beta decay) to 239Np (neptunium), whose half-life is 2.36 days. 239Np decays (negative beta decay) to 239Pu.
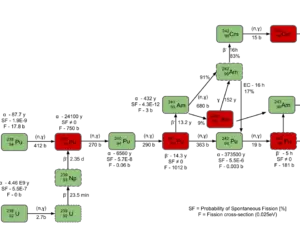
Higher isotopes of plutonium (240Pu, 241Pu and 242Pu) are created by also by neutron radiative capture, but in this case an absorber must be the plutonium nucleus. For example, 240Pu which is the second most common isotope, is formed by radiative capture of a neutron by 239Pu.The transmutation and decay chain is shown below.
Plutonium in Commercial Power Reactors
The nuclear transmutation of 238U into fissile isotopes of plutonium (the plutonium breeding) in the fuel cycle of all commercial light water reactors plays a significant role. In recent years, the commercial power industry has been emphasizing high-burnup fuels (up to 60 – 70 GWd/tU), which are typically enriched to higher percentages of 235U (up to 5%). As burnup increases, a higher percentage of the total power produced in a reactor is due to the plutonium bred inside the reactor.
At a burnup of 30 GWd/tU (gigawatt-days per metric ton of uranium), about 30% of the total energy released comes from bred plutonium. At 40 GWd/tU, that percentage increases to about forty percent. This corresponds to a breeding ratio for these reactors of about 0.4 to 0.5. That means, about half of the fissile fuel in these reactors is bred there. This effect extends the cycle length for such fuels to sometimes nearly twice what it would be otherwise. MOX fuel has a smaller breeding effect than 235U fuel and is thus more challenging and slightly less economic to use due to a quicker drop off in reactivity through cycle life.
Isotopes of Plutonium
About twenty plutonium isotopes have been discovered and described. Except 244Pu, all these isotopes are artificial isotopes. The main isotopes, which have to be considered in the fuel cycle of all commercial light water reactors, are:
- 238Pu. 238Pu belongs to the group of fertile isotopes. 238Pu decays via alpha decay to 234U with half-life of 87.7 years. 238Pu generates very high decay heat and has very high rate of spontaneous fission.
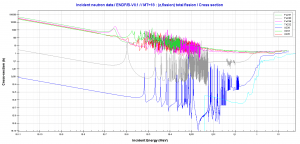
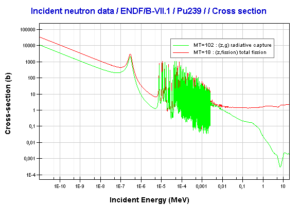
- 239Pu. 239Pu belongs to the group of fissile isotopes. 239Pu decays via alpha decay to 235U with half-life of 24100 years. This isotope is the principal fissile isotope in use.
- 240Pu. 240Pu belongs to the group of fertile isotopes. 240Pu decays via alpha decay to 236U with half-life of 6560 years. 240Pu has very high rate of spontaneous fission and has high radiative capture cross-section for thermal and also for resonance neutrons.
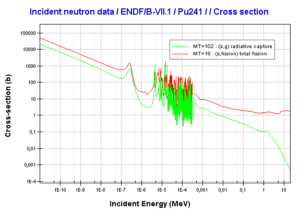
- 241Pu. 241Pu belongs to the group of fissile isotopes. 241Pu decays via negative beta decay to 241Am with half-life of 14.3 years. This fissile isotope decays to non-fissile isotope with high radiative capture cross-section for thermal neutrons. An impact on reactivity of the nuclear fuel is obvious.
- 242Pu. 242Pu belongs to the group of non-fissile isotopes. 242Pu decays via alpha decay to 238U with half-life of 37300 years. 242Pu has very high rate of spontaneous fission but its quantity in the irradiated nuclear fuel is relatively low.
Half-lifes of Isotopes of Plutonium
| Isotope | Half-life / Decay mode | Product |
|---|---|---|
| 238Pu | 87.7 y / alpha decay | 234U |
| 239Pu | 24 100 y / alpha decay | 235U |
| 240Pu | 6 560 y / alpha decay | 236U |
| 241Pu | 14.3 y / beta decay | 241Am |
| 242Pu | 373 500 y / alpha decay | 238U |
| 243Pu | 4.96 d / beta decay | 243Am |
| 244Pu | 80 000 000 y / alpha decay | 240Pu |
Isotope
We hope, this article, Plutonium, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.