This article contains comparison of key thermal and atomic properties of cerium and samarium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Cerium vs Samarium.
Cerium and Samarium – About Elements


Source: www.luciteria.com
Cerium and Samarium – Applications
Cerium
Cerium is an important component of mischmetal alloy. Ferrocerium is a synthetic pyrophoric alloy that produces hot sparks that can reach temperatures of 3,000 °C (5,430 °F) when rapidly oxidized by the process of striking the rod, thereby fragmenting it and exposing those fragments to the oxygen in the air. A typical composition includes approximately 55% cerium, 25% lanthanum, and 15-18% neodymium with other rare earth metals following. The best-known use for this alloy is in ‘flints’ for cigarette lighters. Ceria is the most widely used compound of cerium. The main application of ceria is as a polishing compound, for example in chemical-mechanical planarization (CMP).
Samarium
Samarium is mainly used in preparing samarium-cobalt alloy magnets for electric guitars, small motors and headphones. Samarium-cobalt magnets are much more powerful than iron magnets. They remain magnetic at high temperatures and so are used in microwave applications. They enabled the miniaturisation of electronic devices. However, neodymium magnets are now more commonly used instead. Its oxide is used for manufacturing special infrared adsorbing glass for carbon arc-lamp electrodes. It is useful in doping calcium fluoride crystals employed in optical lasers.
Cerium and Samarium – Comparison in Table
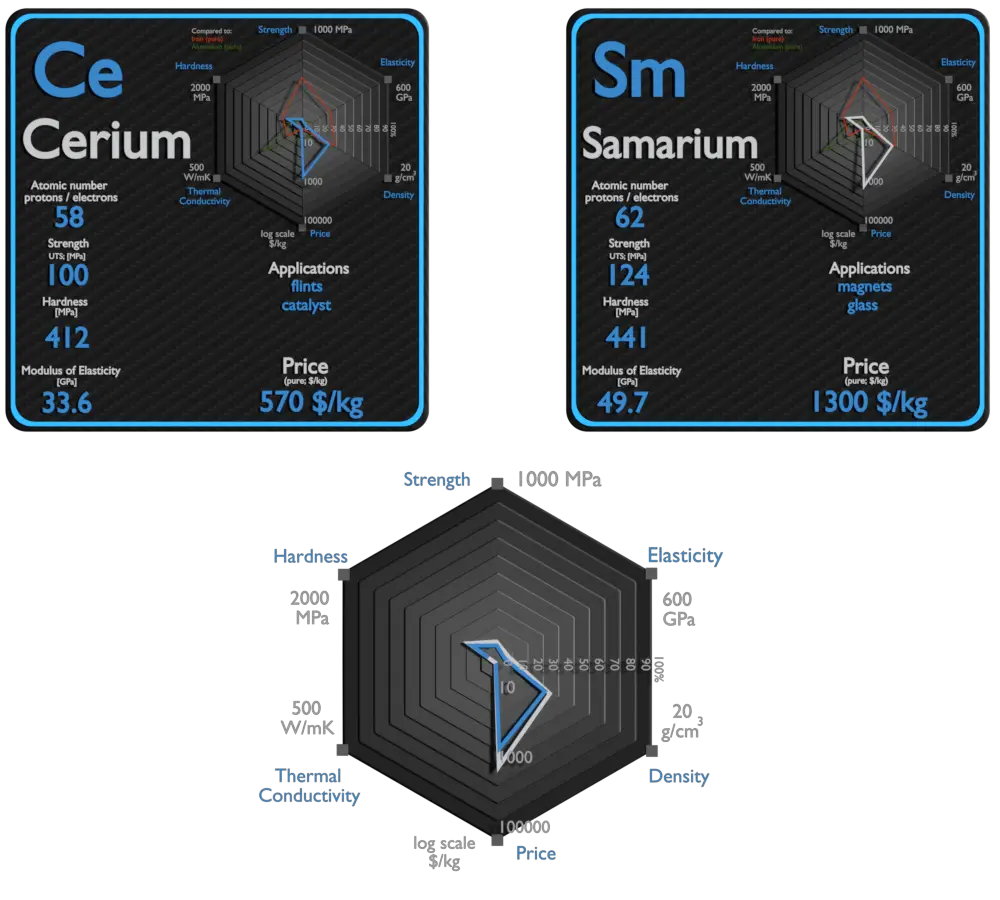
| Element | Cerium | Samarium |
| Density | 6.689 g/cm3 | 7.353 g/cm3 |
| Ultimate Tensile Strength | 100 MPa | 124 MPa |
| Yield Strength | 90 MPa | 110 MPa |
| Young’s Modulus of Elasticity | 33.6 GPa | 49.7 GPa |
| Mohs Scale | 2.5 | N/A |
| Brinell Hardness | 412 MPa | 441 MPa |
| Vickers Hardness | 300 MPa | 412 MPa |
| Melting Point | 798 °C | 1074 °C |
| Boiling Point | 3457 °C | 1900 °C |
| Thermal Conductivity | 11 W/mK | 13 W/mK |
| Thermal Expansion Coefficient | 6.3 µm/mK | 12.7 µm/mK |
| Specific Heat | 0.19 J/g K | 0.2 J/g K |
| Heat of Fusion | 5.46 kJ/mol | 8.63 kJ/mol |
| Heat of Vaporization | 414 kJ/mol | 192 kJ/mol |



