An alloy is a mixture of two or more materials, at least one of which is a metal. Alloys can have a microstructure consisting of solid solutions, where secondary atoms are introduced as substitutionals or interstitials in a crystal lattice. An alloy may also be a mixture of metallic phases (two or more solutions, forming a microstructure of different crystals within the metal). Examples of substitutional alloys include bronze and brass, in which some of the copper atoms are substituted with either tin or zinc atoms respectively.
Solid solutions have important commercial and industrial applications, as such mixtures often have superior properties to pure materials. Many metal alloys are solid solutions. Even small amounts of solute can affect the electrical and physical properties of the solvent.
Alloying is a common practice because metallic bonds allow joining of different types of metals. For example, austenitic stainless steels, including Type 304 stainless steel (containing 18%-20% chromium and 8%-10.5% nickel), have a face-centered cubic structure of iron atoms with the carbon in interstitial solid solution.
Characteristics of Metal Alloys
 Alloys are usually stronger than pure metals, although they generally offer reduced electrical and thermal conductivity. Strength is the most important criterion by which many structural materials are judged. Therefore, alloys are used for engineering construction. Steel, probably the most common structural metal, is a good example of an alloy. It is an alloy of iron and carbon, with other elements to give it certain desirable properties.
Alloys are usually stronger than pure metals, although they generally offer reduced electrical and thermal conductivity. Strength is the most important criterion by which many structural materials are judged. Therefore, alloys are used for engineering construction. Steel, probably the most common structural metal, is a good example of an alloy. It is an alloy of iron and carbon, with other elements to give it certain desirable properties.
It is sometimes possible for a material to be composed of several solid phases. The strengths of these materials are enhanced by allowing a solid structure to become a form composed of two interspersed phases. When the material in question is an alloy, it is possible to quench the metal from a molten state to form the interspersed phases. The term quenching refers to a heat treatment in which a material is rapidly cooled in water, oil or air to obtain certain material properties, especially hardness. In metallurgy, quenching is most commonly used to harden steel by introducing martensite.
Types of Alloys
Metal alloys, by virtue of composition, are often grouped into two classes:
- Ferrous alloys. Ferrous alloys, those in which iron is the principal constituent, include steel, stainless steel, carbon steel, cast iron. Ferrous alloys are known for their strength.
- Non-ferrous alloys. Non-ferrous alloys those that does not contain iron (ferrite) in appreciable amounts thus they are based on non-ferrous metals (i.e. aluminium, copper, chromium, titanium, gold, nickel, silver, tin, lead, zinc, etc.) Other common properties of non-ferrous metals are non-magnetic, malleable, and lightweight.
Ferrous Alloys
 Ferrous alloys, those in which iron is the principal constituent, include steel and pig iron (with a carbon content of a few percent) and alloys of iron with other metals (such as stainless steel). Ferrous alloys are known for their strength. Alloys are usually stronger than pure metals, although they generally offer reduced electrical and thermal conductivity. The simplest ferrous alloys are known as steels and they consist of iron (Fe) alloyed with carbon (C) (about 0.1% to 1%, depending on type). Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater strength. Due to its very-high strength, but still substantial toughness, and its ability to be greatly altered by heat treatment, steel is one of the most useful and common ferrous alloy in modern use. Their widespread use is accounted for by following factors:
Ferrous alloys, those in which iron is the principal constituent, include steel and pig iron (with a carbon content of a few percent) and alloys of iron with other metals (such as stainless steel). Ferrous alloys are known for their strength. Alloys are usually stronger than pure metals, although they generally offer reduced electrical and thermal conductivity. The simplest ferrous alloys are known as steels and they consist of iron (Fe) alloyed with carbon (C) (about 0.1% to 1%, depending on type). Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater strength. Due to its very-high strength, but still substantial toughness, and its ability to be greatly altered by heat treatment, steel is one of the most useful and common ferrous alloy in modern use. Their widespread use is accounted for by following factors:
- Iron containing compounds exist in abundant quantities within the Earth’s crust.
- Metallic iron and steel alloys may be produced using relatively economical extraction, refining, alloying, and fabrication techniques
- Ferrous alloys are extremely versatile, in that they may be tailored to have a wide range of mechanical and physical properties.
The principal disadvantage of many ferrous alloys is their susceptibility to corrosion. By adding chromium to steel, its resistance to corrosion can be enhanced, creating stainless steel, while adding silicon will alter its electrical characteristics, producing silicon steel.
Types of Ferrous Metals – Classification Based on Composition
- Pig Iron. In general, the pig iron is an intermediate product of the iron industry. Pig iron, known also as crude iron, is produced by the blast furnace process and contains up to 4–5% carbon, with small amounts of other impurities like sulfur, magnesium, phosphorus, and manganese. The high level of carbon makes it relatively weak and brittle. Reducing the amount of carbon to 0.002–2.1% by mass produces steel, which may be up to 1000 times harder than pure iron.
- Wrought Iron. Wrought iron is an iron alloy with very low carbon content (less than 0.08%) with respect to cast iron (2.1% to 4%). The microstructure of wrought iron shows dark slag inclusions in ferrite. It is soft, ductile, magnetic, corrosion-resistant and easily welded. It has high elasticity and tensile strength. It can be heated and reheated and worked into various shapes. Wrought iron is no longer produced on a commercial scale. Many products described as wrought iron, such as guard rails, garden furniture and gates, are actually made of mild steel. For example, the Eiffel Tower is a wrought-iron lattice tower.
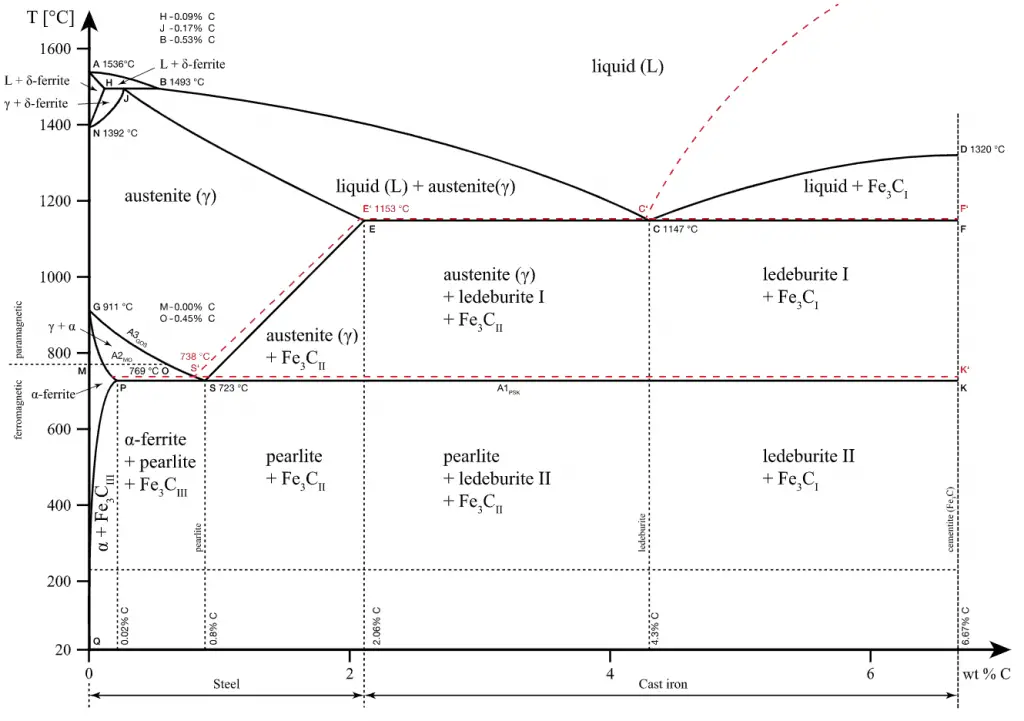
- Steel. Steels are iron–carbon alloys that may contain appreciable concentrations of other alloying elements. Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater strength. Due to its very-high strength, but still substantial toughness, and its ability to be greatly altered by heat treatment, steel is one of the most useful and common ferrous alloy in modern use. There are thousands of alloys that have different compositions and/or heat treatments. The mechanical properties are sensitive to the content of carbon, which is normally less than 1.0 wt%. According ot AISI classification, carbon steel is broken down into four classes based on carbon content:
- Low-carbon Steels. Low-carbon steel, also known as mild steel is now the most common form of steel because its price is relatively low while it provides material properties that are acceptable for many applications. Low-carbon steel contains approximately 0.05–0.25% carbon making it malleable and ductile. Mild steel has a relatively low tensile strength, but it is cheap and easy to form; surface hardness can be increased through carburizing.
- Medium-carbon Steels. Medium-carbon steel has approximately 0.3–0.6% carbon content. Balances ductility and strength and has good wear resistance. This grade of steel is mostly used in the production of machine components, shafts, axles, gears, crankshafts, coupling and forgings and could also be used in rails and railway wheels.
- High-carbon Steels. High-carbon steel has approximately 0.60 to 1.00% carbon content. Hardness is higher than the other grades but ductility decreases. High carbon steels could be used for springs, rope wires, hammers, screwdrivers, and wrenches.
- Ultra-high-carbon Steels. Ultra-high-carbon steel has approximately 1.25–2.0% carbon content. Steels that can be tempered to great hardness. This grade of steel could be used for hard steel products, such as truck springs, metal cutting tools and other special purposes like (non-industrial-purpose) knives, axles or punches. Most steels with more than 2.5% carbon content are made using powder metallurgy.
- Cast Iron. Cast irons also comprise a large family of different types of iron, depending on how the carbon-rich phase forms during solidification. The microstructure of cast irons can be controlled to provide products that have excellent ductility, good machinability, excellent vibration damping, superb wear resistance, and good thermal conductivity. The most common cast iron types are:
- Gray cast iron. Grey cast iron is the oldest and most common type of cast iron. Grey cast iron is characterised by its graphitic microstructure, which causes fractures of the material to have a grey appearance.
- White cast iron. White cast irons are hard, brittle, and unmachinable, while gray irons with softer graphite are reasonably strong and machinable. A fracture surface of this alloy has a white appearance, and thus it is termed white cast iron.
- Malleable cast iron. Malleable cast iron is white cast iron that has been annealed. Through an annealing heat treatment, the brittle structure as first cast is transformed into the malleable form.
- Ductile cast iron. Ductile iron, also known as nodular iron, is very similar to gray iron in composition, ductile iron is stronger and more shock resistant than gray iron. In fact, ductile iron has mechanical characteristics approaching those of steel, while it retains high fluidity when molten and lower melting point.
- Alloy Steels. Steel is an alloy of iron and carbon, but the term alloy steel usually only refers to steels that contain other elements— like vanadium, molybdenum, or cobalt—in amounts sufficient to alter the properties of the base steel. In general, alloy steel is steel that is alloyed with a variety of elements in total amounts between 1.0% and 50% by weight to improve its mechanical properties. Alloy steels are broken down into two groups:
- Low-alloy Steels.
- High-alloy Steels.
- Stainless Steel. Stainless steels are defined as low-carbon steels with at least 10% chromium with or without other alloying elements. Strength and corrosion resistance often make it the material of choice in transportation and processing equipment, engine parts, and firearms. Chromium increases hardness, strength, and corrosion resistance. Nickel gives similar benefits but adds hardness without sacrificing ductility and toughness. It also reduces thermal expansion for better dimensional stability.
- Superalloys.
Special Ferrous Metals
- Tool Steels
- High-speed Steels
- Shock-resisting Steels
- Silver Steel
We hope, this article, Alloys – Composition, Properties of Metal Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.