Intermolecular bonds
Intermolecular forces are the forces which mediate interaction between molecules, including forces of attraction or repulsion which act between molecules and other types of neighboring particles. Attractive intermolecular forces nad their resulting bonds are categorized into the following types:
- Molecular bond. When the electrons of neutral atoms spend more time in one region of their orbit, a temporary weak charge will exist. The molecule will weakly attract other molecules. This is sometimes called the van der Waals or molecular bonds.
- Hydrogen bond. A hydrogen bond can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The hydrogen bond is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative atom or group.
Hydrogen Bond
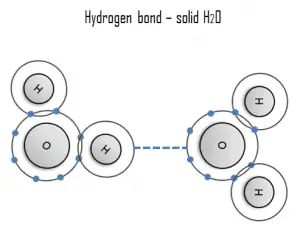 A hydrogen bond can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The hydrogen bond is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative atom or group.
A hydrogen bond can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The hydrogen bond is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative atom or group.
A hydrogen is a strong example of an interaction between two permanent dipoles. The large difference in electronegativities between hydrogen and any of fluorine, nitrogen and oxygen, coupled with their lone pairs of electrons, cause strong electrostatic forces between molecules. A ubiquitous example of a hydrogen bond is found between water molecules. Hydrogen bonds are responsible for the high boiling points of water. Each H2O molecule has two hydrogen atoms that can bond to oxygen atoms. In addition, its single O atom can bond to two hydrogen atoms of other H2O molecules. Thus, for solid ice, each water molecule participates in four hydrogen bonds, helping to create an open hexagonal lattice. Liquid water’s high boiling point is due to the high number of hydrogen bonds each molecule can form, relative to its low molecular mass. Owing to the difficulty of breaking these bonds, water has a very high boiling point, melting point, and viscosity compared to otherwise similar liquids not conjoined by hydrogen bonds.
We hope, this article, Intermolecular Bonds, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.