A chemical bond is a lasting attraction between these atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds. Therefore, the electromagnetic force plays a major role in determining the internal properties of most objects encountered in daily life.
Ionic Bond
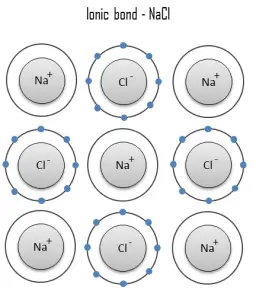 An ionic bond is a chemical bond, in which one or more electrons are wholly transferred from an atom of one element to the atom of the other, and the elements are held together by the force of attraction due to the opposite polarity of the charge. This type of chemical bond is typical between elements with a large electronegativity difference (i.e. elements situated at the horizontal extremities of the periodic table). An ionic bond is always found in compounds composed of both metallic and nonmetallic elements. There is no precise value that distinguishes ionic from covalent bonding, but an electronegativity difference of over 1.7 is likely to be ionic while a difference of less than 1.7 is likely to be covalent.
An ionic bond is a chemical bond, in which one or more electrons are wholly transferred from an atom of one element to the atom of the other, and the elements are held together by the force of attraction due to the opposite polarity of the charge. This type of chemical bond is typical between elements with a large electronegativity difference (i.e. elements situated at the horizontal extremities of the periodic table). An ionic bond is always found in compounds composed of both metallic and nonmetallic elements. There is no precise value that distinguishes ionic from covalent bonding, but an electronegativity difference of over 1.7 is likely to be ionic while a difference of less than 1.7 is likely to be covalent.
Ionic bonding leads to separate positive and negative ions. In the process, all the atoms acquire stable or inert gas configurations (i.e., completely filled orbital shells) and, in addition, an electrical charge – that is, they become ions. For example, common table salt is sodium chloride. Sodium chloride (NaCl) is the classic ionic material. A sodium atom can assume the electron structure of neon by a transfer of its one valence 3s electron to a chlorine atom. After such a transfer, the chlorine ion acquires a net negative charge, an electron configuration identical to that of argon; it is also larger than the chlorine atom. These ions are then attracted to each other in a 1:1 ratio to form sodium chloride (NaCl).
Na + Cl → Na+ + Cl− → NaCl
Ionic compounds conduct electricity when molten or in solution, typically as a solid. Ionic compounds generally have a high melting point, depending on the charge of the ions they consist of. The higher the charges the stronger the cohesive forces and the higher the melting point. They also tend to be soluble in water; the stronger the cohesive forces, the lower the solubility.
We hope, this article, Ionic Bond, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.