 Aluminium alloys are based on aluminium, in which the main alloying elements are Cu, Mn, Si, Mg, Mg+Si, Zn. Aluminium alloys containing alloying elements with limited solid solubility at room temperature and with a strong temperature dependence of solid solubility (for example Cu) can be strengthened by a suitable thermal treatment (precipitation hardening). The strength of heat treated commercial Al alloys exceeds 550 MPa.
Aluminium alloys are based on aluminium, in which the main alloying elements are Cu, Mn, Si, Mg, Mg+Si, Zn. Aluminium alloys containing alloying elements with limited solid solubility at room temperature and with a strong temperature dependence of solid solubility (for example Cu) can be strengthened by a suitable thermal treatment (precipitation hardening). The strength of heat treated commercial Al alloys exceeds 550 MPa.
Mechanical properties of aluminium alloys highly depend on their phase composition and microstructure. High strength can be achieved among others by introduction of a high volume fraction of fine, homogeneously distributed second phase particles and by a refinement of the grain size. In general, aluminium alloys are characterized by a relatively low density (2.7 g/cm3 as compared to 7.9 g/cm3 for steel), high electrical and thermal conductivities, and a resistance to corrosion in some common environments, including the ambient atmosphere. The chief limitation of aluminum is its low melting temperature (660°C), which restricts the maximum temperature at which it can be used.
Strengthening Mechanisms of Aluminium Alloys
The strength of aluminum alloys can be modified through various combinations of cold working, alloying, and heat treating. For example, a microstructure with finer grains typically results in both higher strength and superior toughness compared to the same alloy with physically larger grains. In case of grain size, there may also be tradeoff between strength and creep characteristics. Other strengthening mechanisms are achieved at the expense of lower ductility and toughness.
- Solid Solution Hardening (alloying). Atoms of different elements dissolved in the matrix phase can lead to its strengthening by solid solution strengthening. The solute may incorporate into the solvent crystal lattice substitutionally, by replacing a solvent particle in the lattice, or interstitially, by fitting into the space between solvent particles. This impose lattice strains on surrounding atoms resulting in a lattice strain field. Even small amounts of solute can affect the electrical and physical properties of the solvent. Manganese and magnesium are examples of elements added to aluminum for the purpose of strengthening. Solid solution strengthening occurs in 3xxx and 5xxx alloys through the addition of manganese (3xxx) and magnesium (5xxx) to aluminum.
- Strain Hardening (cold working). Strain hardening is also called work-hardening or cold-working is a strengthening method often used in materials whose strength cannot be increased by heat treatment, e.g. by changes in their phase composition. It is called cold-working because the plastic deformation must occurs at a temperature low enough that atoms cannot rearrange themselves. It is a process of making a metal harder and stronger through plastic deformation. When a metal is plastically deformed, dislocations move and additional dislocations are generated. Dislocations can move if the atoms from one of the surrounding planes break their bonds and rebond with the atoms at the terminating edge. The dislocation density in a metal increases with deformation or cold work because of dislocation multiplication or the formation of new dislocations. The more dislocations within a material, the more they will interact and become pinned or tangled. This will result in a decrease in the mobility of the dislocations and a strengthening of the material. Cold working involves the reduction in thickness of a material. Plate and sheet of different thickness are produced by cold rolling. Wire and tubes of different diameter and wall thickness are produced by drawing. All aluminum alloys can be strengthened by cold working.
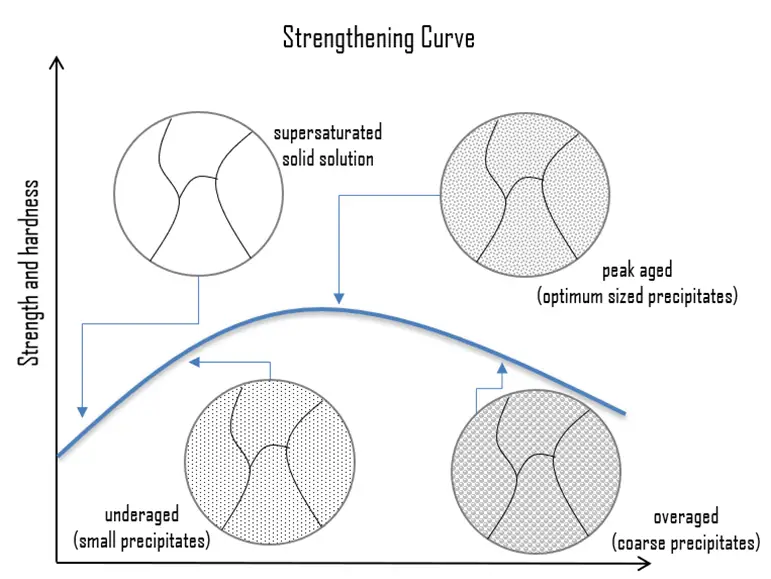 Precipitation (age) Hardening. Precipitation hardening, also called age hardening or particle hardening, is a heat treatment technique based on the formation of extremely small, uniformly dispersed particles (precipitates) of a second phase within the original phase matrix to enhance the strength and hardness of some metal alloys. Second phase particles present further type of obstacles for dislocation movement. The presence of a second phase particle represents a distortion in the matrix lattice. Therefore, the obstacles which hinder the dislocation motion are either the strain field around second phase particles or the second phase particles itself or both. Precipitation hardening is used to increase the yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nickel, titanium, and some steels and stainless steels. In superalloys, it is known to cause yield strength anomaly providing excellent high-temperature strength. In case of aluminium alloys, precipitation strengthening can increase the yield strength of aluminium from about five times up to about fifteen times that of unalloyed aluminium. Especially 2xxx series, which are alloyed with copper, can be precipitation hardened to strengths comparable to steel. In terms of age hardening, solution annealed aluminum-copper alloys can be aged naturally at room temperature for four days or more to obtain maximum properties such as hardness and strength. This process is known as natural aging. The aging process also can be accelerated to a matter of hours after solution treatment and quenching by heating the supersaturated alloy to a specific temperature and holding at that temperature for a specified time. This process is called artificial aging.
Precipitation (age) Hardening. Precipitation hardening, also called age hardening or particle hardening, is a heat treatment technique based on the formation of extremely small, uniformly dispersed particles (precipitates) of a second phase within the original phase matrix to enhance the strength and hardness of some metal alloys. Second phase particles present further type of obstacles for dislocation movement. The presence of a second phase particle represents a distortion in the matrix lattice. Therefore, the obstacles which hinder the dislocation motion are either the strain field around second phase particles or the second phase particles itself or both. Precipitation hardening is used to increase the yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nickel, titanium, and some steels and stainless steels. In superalloys, it is known to cause yield strength anomaly providing excellent high-temperature strength. In case of aluminium alloys, precipitation strengthening can increase the yield strength of aluminium from about five times up to about fifteen times that of unalloyed aluminium. Especially 2xxx series, which are alloyed with copper, can be precipitation hardened to strengths comparable to steel. In terms of age hardening, solution annealed aluminum-copper alloys can be aged naturally at room temperature for four days or more to obtain maximum properties such as hardness and strength. This process is known as natural aging. The aging process also can be accelerated to a matter of hours after solution treatment and quenching by heating the supersaturated alloy to a specific temperature and holding at that temperature for a specified time. This process is called artificial aging.- Dispersion Hardening. Dispersion hardening involves the inclusion of small, hard particles in the metal, thus restricting the movement of dislocations, and thereby raising the strength properties. It is, in many ways, very similar to age hardening. The difference lies in the precipitates themselves—the particles are chosen because of their thermal stability, that is, their resistance to particle coarsening or growth at high temperatures. Dispersoid particles influence the grain structure. The increase in strength is due to the grain structure formed as a result of the presence of dispersoids.
- Grain Refinement (small grain size). The size of the grain determines the properties of the metal. For example, smaller grain size increases tensile strength and tends to increase ductility. A larger grain size is preferred for improved high-temperature creep properties. Decreasing the grain size also is an effective way to increase ductility. When grain size is reduced, there are more grains with a greater number of arbitrarily aligned slip planes for the dislocations in the grains. This provides more opportunity for some slip to occur in a stressed material. Thus, grain refinement provides an important means to improve not only strength, but also ductility and toughness. Many other strengthening mechanisms are achieved at the expense of ductility and toughness.
We hope, this article, Strengthening Mechanisms of Aluminium Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.