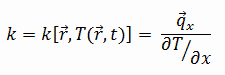The bronzes are a family of copper-based alloys traditionally alloyed with tin, but can refer to alloys of copper and other elements (e.g. aluminum, silicon, and nickel). Bronzes are somewhat stronger than the brasses, yet they still have a high degree of corrosion resistance. Generally they are used when, in addition to corrosion resistance, good tensile properties are required. For example, beryllium copper attains the greatest strength (to 1,400 MPa) of any copper-based alloy.
The bronzes are a family of copper-based alloys traditionally alloyed with tin, but can refer to alloys of copper and other elements (e.g. aluminum, silicon, and nickel). Bronzes are somewhat stronger than the brasses, yet they still have a high degree of corrosion resistance. Generally they are used when, in addition to corrosion resistance, good tensile properties are required. For example, beryllium copper attains the greatest strength (to 1,400 MPa) of any copper-based alloy.
Historically, alloying copper with another metal, for example tin to make bronze, was first practiced about 4000 years after the discovery of copper smelting, and about 2000 years after “natural bronze” had come into general use. An ancient civilization is defined to be in the Bronze Age either by producing bronze by smelting its own copper and alloying with tin, arsenic, or other metals. Bronze, or bronze-like alloys and mixtures, were used for coins over a longer period. is still widely used today for springs, bearings, bushings, automobile transmission pilot bearings, and similar fittings, and is particularly common in the bearings of small electric motors. Brass and bronze are common engineering materials in modern architecture and primarily used for roofing and facade cladding due to their visual appearance.
Thermal Properties of Bronzes
Thermal properties of materials refer to the response of materials to changes in their temperature and to the application of heat. As a solid absorbs energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are properties that are often critical in the practical use of solids.
Melting Point of Bronzes
Melting point of aluminium bronze – UNS C95400 is around 1030°C.
Melting point of tin bronze – UNS C90500 – gun metal is around 1000°C.
Melting point of copper beryllium – UNS C17200 is around 866°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Bronzes
The thermal conductivity of aluminium bronze – UNS C95400 is 59 W/(m.K).
The thermal conductivity of tin bronze – UNS C90500 – gun metal is 75 W/(m.K).
The thermal conductivity of copper beryllium – UNS C17200 is 115 W/(m.K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
We hope, this article, Thermal Properties of Bronzes, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.