Discovery of Photoelectric Effect
While this is interesting, it is hardly explainable by classical theory of electromagnetic radiation which assumed the existence of a stationary medium (the luminiferous aether) through which light propagated. Subsequent investigations into the photoelectric effect results in the fact that these explorations did not fit with the classical theory of electromagnetic radiation.In 1905, Albert Einstein published four groundbreaking papers on the photoelectric effect, Brownian motion, special relativity, and the equivalence of mass and energy. These papers were published in the Annalen der Physik journal and contributed significantly to the foundation of modern physics. In the paper on the photoelectric effect (“On a Heuristic Viewpoint Concerning the Production and Transformation of Light”) he solved the paradox by describing light as composed of discrete quanta (German: das Lichtquant), rather than continuous waves.This theory was builded on Max Planck’s blackbody radiation theory, which assumes that luminous energy can be absorbed or emitted only in discrete amounts, called quanta. The photon’s energy in each quantum of light is equal to its frequency (ν) multiplied by a constant known as Planck’s constant (h), or alternately, using the wavelength (λ) and the speed of light (c):
E=hc/λ=hν
Each photon above a threshold frequency (specific for each material) has the needed energy to eject a single electron, creating the observed effect. Einstein’s theory predicts that the maximum kinetic energy of emitted electron is dependent only on the frequency of the incident light and not on its intensity. Shining twice as much light (high-intensity) results in twice as many photons, and more electrons releasing, but the maximum kinetic energy of those individual electrons remains the same. Experimentation in the photoelectric effect was carried out extensively by Robert Millikan in 1915, Robert Millikan showed that Einstein’s prediction was correct. This discovery contributed to the quantum revolution in physics and earned Einstein the Nobel Prize in Physics in 1921.
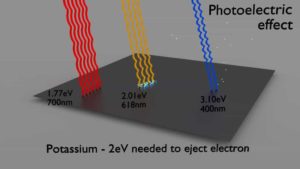
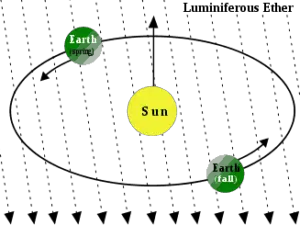
Source: wikipedia.org
We hope, this article, Albert Einstein and Photoelectric Effect, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.