 Chloride stress corrosion occurs in austenitic stainless steels under tensile stress in the presence of oxygen, chloride ions, and high temperature. It is one of the most important forms of stress corrosion that concerns the nuclear industry. Austenitic stainless steels contain between 16 and 25% Cr and can also contain nitrogen in solution, both of which contribute to their relatively high uniform corrosion resistance. One type of corrosion which can attack austenitic stainless steel is chloride stress corrosion.
Chloride stress corrosion occurs in austenitic stainless steels under tensile stress in the presence of oxygen, chloride ions, and high temperature. It is one of the most important forms of stress corrosion that concerns the nuclear industry. Austenitic stainless steels contain between 16 and 25% Cr and can also contain nitrogen in solution, both of which contribute to their relatively high uniform corrosion resistance. One type of corrosion which can attack austenitic stainless steel is chloride stress corrosion.
The three conditions that must be present for chloride stress corrosion to occur are as follows:
- Chloride ions are present in the environment
- Dissolved oxygen is present in the environment
- Metal is under tensile stress
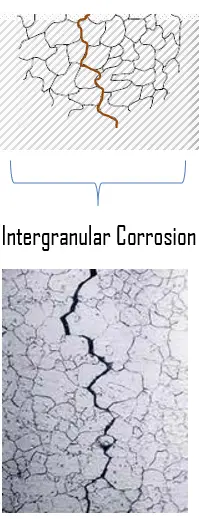
Chloride stress corrosion involves selective attack of the metal along grain boundaries. The resistance of these metallic alloys to the chemical effects of corrosive agents is based on passivation. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 10.5% by weight, above which passivity can occur and below which it is impossible. But the chromium carbides may precipitate in the grain boundaries, which result in depletion of chromium in the zones close to the grain boundaries due to the diffusion rate of chromium that is slow. The chromium-depleted zones become less corrosion resistant than the rest of the matrix. In a corrosive environment the depleted areas may be activated and corrosion will occur in very narrow areas between the grains.
It has been found that this is closely associated with certain heat treatments resulting from welding. This can be minimized considerably by proper annealing processes. This form of corrosion is controlled by maintaining low chloride ion and oxygen content in the environment and use of low carbon steels. Ferritic stainless steels are chosen for their resistance to stress corrosion cracking, which makes them an attractive alternative to austenitic stainless steels in applications where chloride-induced SCC is prevalent.
We hope, this article, Chloride Stress Corrosion Cracking, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.