Corrosion is the deterioration of a material due to chemical interaction with its environment. It is natural process in which metals convert its structure into a more chemically-stable form such as oxides, hydroxides, or sulfides. The consequences of corrosion are all too common. Familiar examples include the rusting of automotive body panels and pipings and many tools. Corrosion is usually a negative phenomenon, since it is associated with mechanical failure of an object. Metal atoms are removed from a structural element until it fails, or oxides build up inside a pipe until it is plugged. All metals and alloys are subject to corrosion. Even the noble metals, such as gold, are subject to corrosive attack in some environments.
Selective Leaching – Selective Corrosion
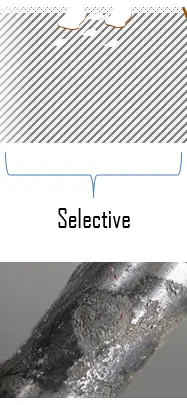 Selective leaching or selective corrosion is the removal of one element from a solid alloy by corrosion process. The most common example is the dezincification of brass, in which zinc is selectively leached from a copper–zinc brass alloy. This process produces a weakened porous copper structure. The selective removal of zinc can be in a uniform manner or localized scale.
Selective leaching or selective corrosion is the removal of one element from a solid alloy by corrosion process. The most common example is the dezincification of brass, in which zinc is selectively leached from a copper–zinc brass alloy. This process produces a weakened porous copper structure. The selective removal of zinc can be in a uniform manner or localized scale.
However, many alloys are subject to selective leaching under certain conditions. Similar process occurs in other alloy systems in which aluminum, iron, cobalt, chromium, and other elements are removed. Elements in an alloy that are more resistant to the environment remain behind. Two mechanisms are involved:
- Two metals in an alloy are dissolved; one metal redeposits on the surface of the surviving elements.
- One metal is selectively dissolved, leaving the other metals behind.
The first system is involved in the dezincification of brasses, and the second system is involved when molybdenum is removed from nickel alloys in molten sodium hydroxide.
We hope, this article, Selective Leaching – Selective Corrosion, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.