 Titanium is a lustrous transition metal with a silver color, low density, and high strength. Titanium is resistant to corrosion in sea water, aqua regia, and chlorine. In power plants, titanium can be used in surface condensers. Pure titanium is stronger than common, low-carbon steels, but 45% lighter. It is also twice as strong as weak aluminium alloys but only 60% heavier. The two most useful properties of the metal are corrosion resistance and strength-to-density ratio, the highest of any metallic element.
Titanium is a lustrous transition metal with a silver color, low density, and high strength. Titanium is resistant to corrosion in sea water, aqua regia, and chlorine. In power plants, titanium can be used in surface condensers. Pure titanium is stronger than common, low-carbon steels, but 45% lighter. It is also twice as strong as weak aluminium alloys but only 60% heavier. The two most useful properties of the metal are corrosion resistance and strength-to-density ratio, the highest of any metallic element.
Density of Titanium Alloys
Density of typical titanium alloy is 4.43 g/cm3 (Ti-6Al-4V).
In comparison to other common materials:
Density of typical aluminium alloy is 2.7 g/cm3 (6061 alloy).
Density of typical magnesium alloy is 1.8 g/cm3 (Elektron 21).
Density of typical stainless steel is 8.0 g/cm3 (AISI 304L).
Density is defined as the mass per unit volume. It is an intensive property, which is mathematically defined as mass divided by volume:
ρ = m/V
In words, the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance. The standard SI unit is kilograms per cubic meter (kg/m3). The Standard English unit is pounds mass per cubic foot (lbm/ft3).
Since the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance, it is obvious, the density of a substance strongly depends on its atomic mass and also on the atomic number density (N; atoms/cm3),
- Atomic Weight. The atomic mass is carried by the atomic nucleus, which occupies only about 10-12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Therefore it is determined by the mass number (number of protons and neutrons).
- Atomic Number Density. The atomic number density (N; atoms/cm3), which is associated with atomic radii, is the number of atoms of a given type per unit volume (V; cm3) of the material. The atomic number density (N; atoms/cm3) of a pure material having atomic or molecular weight (M; grams/mol) and the material density (⍴; gram/cm3) is easily computed from the following equation using Avogadro’s number (NA = 6.022×1023 atoms or molecules per mole):
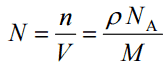
- Crystal Structure. Density of crystalline substance is significantly affected by its crystal structure. FCC structure, along with its hexagonal relative (hcp), has the most efficient packing factor (74%). Metals containing FCC structures include austenite, aluminum, copper, lead, silver, gold, nickel, platinum, and thorium.
We hope, this article, Density of Titanium Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.