 Titanium is a lustrous transition metal with a silver color, low density, and high strength. Titanium is resistant to corrosion in sea water, aqua regia, and chlorine. In power plants, titanium can be used in surface condensers. The metal is extracted from its principal mineral ores by the Kroll and Hunter processes. Kroll’s process involved reduction of titanium tetrachloride (TiCl4), first with sodium and calcium, and later with magnesium, under an inert gas atmosphere. Pure titanium is stronger than common, low-carbon steels, but 45% lighter. It is also twice as strong as weak aluminium alloys but only 60% heavier. The two most useful properties of the metal are corrosion resistance and strength-to-density ratio, the highest of any metallic element. The corrosion resistance of titanium alloys at normal temperatures is unusually high. Titanium’s corrosion resistance is based on the formation of a stable, protective oxide layer. Although “commercially pure” titanium has acceptable mechanical properties and has been used for orthopedic and dental implants, for most applications titanium is alloyed with small amounts of aluminium and vanadium, typically 6% and 4% respectively, by weight. This mixture has a solid solubility which varies dramatically with temperature, allowing it to undergo precipitation strengthening.
Titanium is a lustrous transition metal with a silver color, low density, and high strength. Titanium is resistant to corrosion in sea water, aqua regia, and chlorine. In power plants, titanium can be used in surface condensers. The metal is extracted from its principal mineral ores by the Kroll and Hunter processes. Kroll’s process involved reduction of titanium tetrachloride (TiCl4), first with sodium and calcium, and later with magnesium, under an inert gas atmosphere. Pure titanium is stronger than common, low-carbon steels, but 45% lighter. It is also twice as strong as weak aluminium alloys but only 60% heavier. The two most useful properties of the metal are corrosion resistance and strength-to-density ratio, the highest of any metallic element. The corrosion resistance of titanium alloys at normal temperatures is unusually high. Titanium’s corrosion resistance is based on the formation of a stable, protective oxide layer. Although “commercially pure” titanium has acceptable mechanical properties and has been used for orthopedic and dental implants, for most applications titanium is alloyed with small amounts of aluminium and vanadium, typically 6% and 4% respectively, by weight. This mixture has a solid solubility which varies dramatically with temperature, allowing it to undergo precipitation strengthening.
Titanium alloys are metals that contain a mixture of titanium and other chemical elements. Such alloys have very high tensile strength and toughness (even at extreme temperatures). They are light in weight, have extraordinary corrosion resistance and the ability to withstand extreme temperatures.
Application of Titanium Alloys – Uses
The two most useful properties of the metal are corrosion resistance and strength-to-density ratio, the highest of any metallic element. The corrosion resistance of titanium alloys at normal temperatures is unusually high. These properties determine application of titanium and its alloys. The earliest production application of titanium was in 1952, for the nacelles and firewalls of the Douglas DC-7 airliner. High specific strength, good fatigue resistance and creep life, and good fracture toughness are characteristics that make titanium a preferred metal for aerospace applications. Aerospace applications, including use in both structural (airframe) components and jet engines, still account for the largest share of titanium alloy use. On the supersonic aircraft SR-71, titanium was used for 85% of the structure. Due to very high inertness, titanium has many biomedical applications, which is based on its inertness in the human body, that is, resistance to corrosion by body fluids.
Commercially Pure Titanium – Grade 1 in Steam Condensers
In nuclear power plants, the main steam condenser (MC) system is designed to condense and deaerate the exhaust steam from the main turbine and provide a heat sink for the turbine bypass system. The exhausted steam from the LP turbines is condensed by passing over tubes containing water from the cooling system. These tubes are usually made of stainless steel, copper alloys, or titanium depending on several selection criteria (such as thermal conductivity or corrosion resistance). Titanium condenser tubes are usually the best technical choice, however titanium is very expensive material and the use of titanium condenser tubes is associated with very high initial costs. Titanium in particular can bring major improvements such as higher water velocities promoting better heat coefficients, excellent resistance to abrasion, erosion and corrosion thereby improving resistance to fouling. Tubes are mostly welded tubes from ASTM SB 338 grade 1 made on a continuous manufacturing line. This commercially pure titanium is the softest titanium and has the highest ductility. It has good cold forming characteristics and provides excellent corrosion resistance. It also has excellent welding properties and high impact toughness. All manufacturing operations (welding, annealing, non-destructive testing) are fully automated to produce high quality tubes in large quantities.
Titanium Grades
Pure titanium and its alloys is commonly defined by their grades defined by ASTM Internation standard. In general, there are almost 40 grades of titanium and its alloys. Following is an overview of the most frequently encountered titanium alloys and pure grades, their properties, benefits, and industry applications.
 Grade 1. Commercially pure titanium grade 1 is the most ductile and softest titanium alloy. It is a good solution for cold forming and corrosive environments. It possesses the greatest formability, excellent corrosion resistance and high impact toughness. Due to its formability, it is commonly available as titanium plate and tubing. These include:
Grade 1. Commercially pure titanium grade 1 is the most ductile and softest titanium alloy. It is a good solution for cold forming and corrosive environments. It possesses the greatest formability, excellent corrosion resistance and high impact toughness. Due to its formability, it is commonly available as titanium plate and tubing. These include:
- Chemical processing
- Chlorate manufacturing
- Architecture
- Medical industry
- Marine industry
- Automotive parts
- Airframe structure
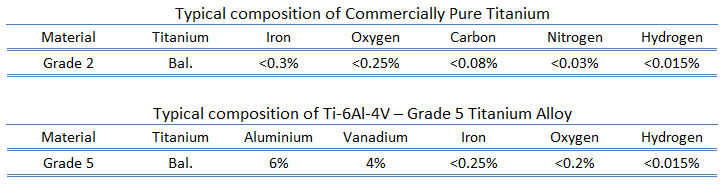
- Grade 2. Commercially pure titanium grade 2 is very similar to grade 1, but it has higher strength than grade 1 and excellent cold forming properties. It provides excellent welding properties and has excellent resistance to oxidation and corrosion. This grade of titanium is the most common grade of the commercially pure titanium industry. It is the prime choice for many fields of applications:
- Aerospace,
- Automotive,
- Chemical Processing & Chlorate Manufacturing,
- Desalination
- Power generation
- Grade 5 – Ti-6Al-4V. Grade 5 is the most commonly used alloy and it is an alpha + beta alloy. Grade 5 alloy accounts for 50% of total titanium usage the world over. It has a chemical composition of 6% aluminum, 4% vanadium, 0.25% (maximum) iron, 0.2% (maximum) oxygen, and the remainder titanium. Generally, Ti-6Al-4V is used in applications up to 400 degrees Celsius. It has a density of roughly 4420 kg/m3. It is significantly stronger than commercially pure titanium (grades 1-4) due to its possibility to be heat treated. This grade is an excellent combination of strength, corrosion resistance, weld and fabricability It is the prime choice for many fields of applications:
- Aircraft turbines
- Engine components
- Aircraft structural components
- Aerospace fasteners
- High-performance automatic parts
- Marine applications
- Grade 23 – Ti-6Al-4V-ELI. Ti-6Al-4V-ELI or TAV-ELI is the higher purity version of Ti-6Al-4V. ELI stands for Extra Low Interstitial. The essential difference between Ti6Al4V ELI (grade 23) and Ti6Al4V (grade 5) is the reduction of oxygen content to 0.13% (maximum) in grade 23. Reduced interstitial elements oxygen and iron improve ductility and fracture toughness with some reduction in strength. It’s the top choice for any sort of situation where a combination of high strength, light weight, good corrosion resistance and high toughness are required. This grade of titanium, medical grade of titanium, is used in biomedical applications such as implantable components due to its biocompatibility, good fatigue strength and low modulus.
We hope, this article, Application of Titanium Alloys – Uses, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.