 Copper alloys are alloys based on copper, in which the main alloying elements are Zn, Sn, Si, Al, Ni. Cu-based alloys constitute mostly substitutional solid solutions, for which solute or impurity atoms replace or substitute for the host atoms. Several features of the solute and solvent atoms determine the degree to which the former dissolves in the latter. These are expressed as the Hume–Rothery rules. There are as many as 400 different copper and copper alloy compositions loosely grouped into the categories: copper, high copper alloy, brasses, bronzes, copper nickels, copper–nickel–zinc (nickel silver), leaded copper, and special alloys. In addition, a limited number of copper alloys can be strengthened by heat treatment.; consequently, cold working and/or solid-solution alloying must be used to improve these mechanical properties.
Copper alloys are alloys based on copper, in which the main alloying elements are Zn, Sn, Si, Al, Ni. Cu-based alloys constitute mostly substitutional solid solutions, for which solute or impurity atoms replace or substitute for the host atoms. Several features of the solute and solvent atoms determine the degree to which the former dissolves in the latter. These are expressed as the Hume–Rothery rules. There are as many as 400 different copper and copper alloy compositions loosely grouped into the categories: copper, high copper alloy, brasses, bronzes, copper nickels, copper–nickel–zinc (nickel silver), leaded copper, and special alloys. In addition, a limited number of copper alloys can be strengthened by heat treatment.; consequently, cold working and/or solid-solution alloying must be used to improve these mechanical properties.
Copper and its alloys has excellent electrical conductivity. The conductivity of copper is 97% that of silver. Due to its much lower cost and greater abundance, copper has traditionally been the standard material used for electricity transmission applications. However, aluminium is usually used in overhead high-voltage power lines because it has about half the weight and lower cost of a comparable resistance copper cable. At a given temperature, the thermal and electrical conductivities of metals are proportional, but raising the temperature increases the thermal conductivity while decreasing the electrical conductivity. This behavior is quantified in the Wiedemann–Franz law.
Types of Copper Alloys
As was written, there are as many as 400 different copper and copper alloy compositions loosely grouped into the categories: copper, high copper alloy, brasses, bronzes, copper nickels, copper–nickel–zinc (nickel silver), leaded copper, and special alloys. In following points we summarize key properties of selected copper-based materials.
 Electrolytic-tough pitch (ETP) copper. Electrolytic tough pitch copper, UNS C11000, is pure copper (with a maximum of 0.0355% of impurities) refined by electrolytic refining process and it is the most widely used grade of copper all over the world. ETP has a minimum conductivity rating of 100% IACS and is required to be 99.9% pure. It has 0.02% to 0.04% oxygen content (typical). Electrical wiring is the most important market for the copper industry. This includes structural power wiring, power distribution cable, appliance wire, communications cable, automotive wire and cable, and magnet wire. Roughly half of all copper mined is used for electrical wire and cable conductors. Pure copper has the best electrical and thermal conductivity of any commercial metal. The conductivity of copper is 97% that of silver. Due to its much lower cost and greater abundance, copper has traditionally been the standard material used for electricity transmission applications.
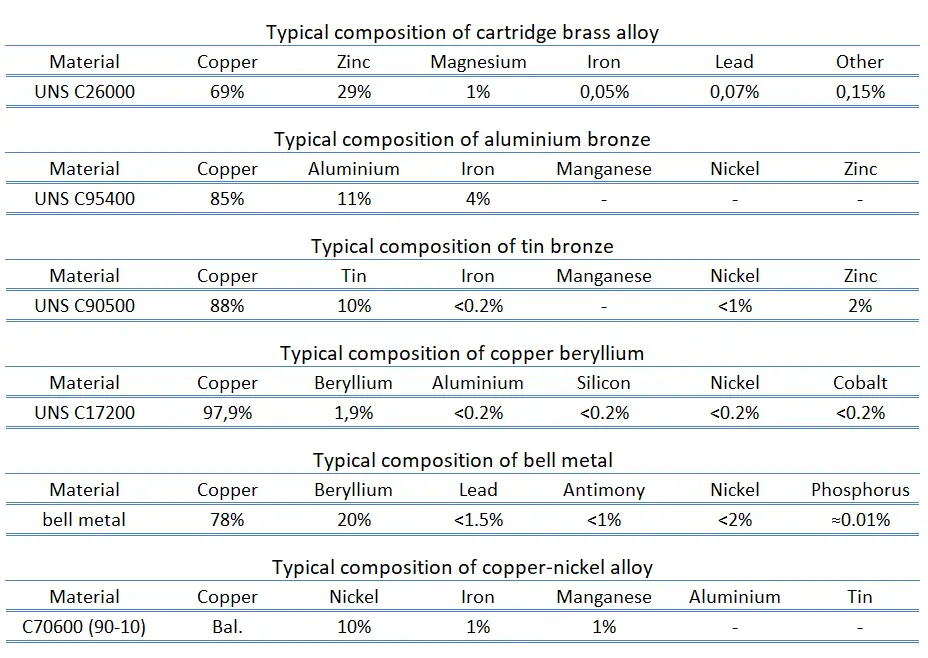
Electrolytic-tough pitch (ETP) copper. Electrolytic tough pitch copper, UNS C11000, is pure copper (with a maximum of 0.0355% of impurities) refined by electrolytic refining process and it is the most widely used grade of copper all over the world. ETP has a minimum conductivity rating of 100% IACS and is required to be 99.9% pure. It has 0.02% to 0.04% oxygen content (typical). Electrical wiring is the most important market for the copper industry. This includes structural power wiring, power distribution cable, appliance wire, communications cable, automotive wire and cable, and magnet wire. Roughly half of all copper mined is used for electrical wire and cable conductors. Pure copper has the best electrical and thermal conductivity of any commercial metal. The conductivity of copper is 97% that of silver. Due to its much lower cost and greater abundance, copper has traditionally been the standard material used for electricity transmission applications.- Brass. Brass is is the generic term for a range of copper-zinc alloys. Brass can be alloyed with zinc in different proportions, which results in a material of varying mechanical, corrosion and thermal properties. Increased amounts of zinc provide the material with improved strength and ductility. Brasses with a copper content greater than 63% are the most ductile of any copper alloy and are shaped by complex cold forming operations. Brass has higher malleability than bronze or zinc. The relatively low melting point of brass and its fluidity make it a relatively easy material to cast. Brass can range in surface color from red to yellow depending on the zinc content. Some of the common uses for brass alloys include costume jewelry, locks, hinges, gears, bearings, hose couplings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins. Brass and bronze are common engineering materials in modern architecture and primarily used for roofing and facade cladding due to their visual appearance.
- Bronze. The bronzes are a family of copper-based alloys traditionally alloyed with tin, but can refer to alloys of copper and other elements (e.g. aluminum, silicon, and nickel). Bronzes are somewhat stronger than the brasses, yet they still have a high degree of corrosion resistance. Generally they are used when, in addition to corrosion resistance, good tensile properties are required. For example, beryllium copper attains the greatest strength (to 1,400 MPa) of any copper-based alloy.
- Copper-nickel Alloy. Cupronickels are copper-nickel alloys that contain typically from 60 to 90 percent of copper and nickel as the main alloying element. The two main alloys are 90/10 and 70/30. Other strengthening elements, such as manganese and iron, may be also contained. Cupronickels have excellent resistance to corrosion caused by sea water. Despite its high copper content, cupronickel is silver in colour. The addition of nickel to copper also improves strength and corrosion resistance, but good ductility is retained.
- Nickel Silver. Nickel silver, known also as German silver, nickel brass or alpacca, is a copper alloy with nickel and often zinc. For example, UNS C75700 nickel silver 65-12 copper alloy has good corrosion and tarnish-resistance, and high formability. Nickel silver is named due to its silvery appearance, but it contains no elemental silver unless plated.
Electrical Conductivity of Copper Alloys
The electrical conductivity of electrolytic-tough pitch (ETP) copper is 101% IACS (around 58.6 MS/m).
The electrical conductivity of carthridge brass – UNS C26000 is about 30% IACS (around 17 MS/m).
Electrical resistivity and its converse, electrical conductivity, is a fundamental property of a material that quantifies how strongly it resists or conducts the flow of electric current. A low resistivity indicates a material that readily allows the flow of electric current. The symbol of resistivity is usually the Greek letter ρ (rho). The SI unit of electrical resistivity is the ohm-metre (Ω⋅m). Note that, electrical resistivity is not the same as electrical resistance. Electrical resistance is expressed in Ohms. While resistivity is a material property, resistance is the property of an object.
We hope, this article, Electrical Conductivity of Copper Alloys, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.