Corrosion is the deterioration of a material due to chemical interaction with its environment. It is natural process in which metals convert its structure into a more chemically-stable form such as oxides, hydroxides, or sulfides. The consequences of corrosion are all too common. Familiar examples include the rusting of automotive body panels and pipings and many tools. Corrosion is usually a negative phenomenon, since it is associated with mechanical failure of an object. Metal atoms are removed from a structural element until it fails, or oxides build up inside a pipe until it is plugged. All metals and alloys are subject to corrosion. Even the noble metals, such as gold, are subject to corrosive attack in some environments.
General Corrosion
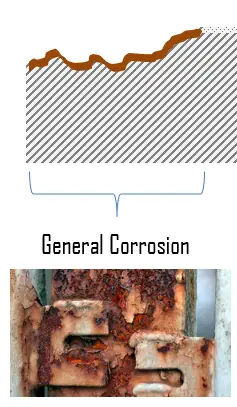 General corrosion, also known as uniform corrosion, is a form of corrosion that affects the entire surface of the metal, whereas other forms affect a specific spot or portion. It is the most common form of corrosion. This type of corrosion is commonly observed in pure metals which are metallurgical and compositionally uniform. Weathering steels, magnesium alloys, zinc alloys, and copper alloys are examples of materials that typically exhibit general corrosion. Passive materials, such as stainless steels, aluminum alloys, or nickelchromium alloys are generally subject to localized corrosion.
General corrosion, also known as uniform corrosion, is a form of corrosion that affects the entire surface of the metal, whereas other forms affect a specific spot or portion. It is the most common form of corrosion. This type of corrosion is commonly observed in pure metals which are metallurgical and compositionally uniform. Weathering steels, magnesium alloys, zinc alloys, and copper alloys are examples of materials that typically exhibit general corrosion. Passive materials, such as stainless steels, aluminum alloys, or nickelchromium alloys are generally subject to localized corrosion.
It is a very slow reaction that is fairly evenly distributed over the entire metal surface exposed to any circulating water. The fact that it affects a fairly large area of the metal makes it much easier to detect, and hence much less severe than localized corrosion. The problem with general corrosion is that it results in a large volume of oxides that tend to attach themselves to the heat transfer surfaces and affect the efficiency of the system.
General Corrosion – Protection
Some standard methods associated with material selection that protect against general corrosion include:
- The use of corrosion-resistant materials such as stainless steel and nickel, chromium, and molybdenum alloys.
- The use of protective coatings such as paints and epoxies.
- The application of metallic and nonmetallic coatings or linings to the surface which protects against corrosion, but allows the material to retain its structural strength (for example, a carbon steel pressure vessel with stainless steel cladding as a liner).
We hope, this article, General Corrosion, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.