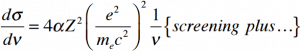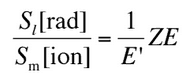Description Beta Particles
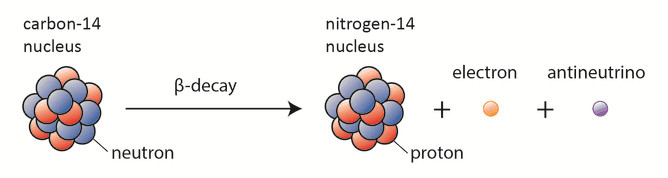
Beta particles are high-energy, high-speed electrons or positrons emitted by certain fission fragments or by certain primordial radioactive nuclei such as potassium-40. The beta particles are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay. There are two forms of beta decay, the electron decay (β− decay) and the positron decay (β+ decay). In a nuclear reactor occurs especially the β− decay, because the common feature of the fission products is an excess of neutrons (see Nuclear Stability). An unstable fission fragment with the excess of neutrons undergoes β− decay, where the neutron is converted into a proton, an electron, and an electron antineutrino.
Spectrum of beta particles
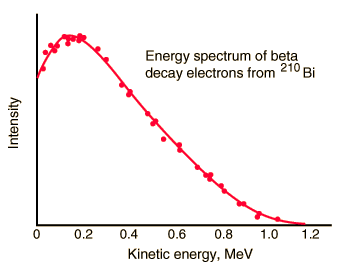
In the process of beta decay, either an electron or a positron is emitted. This emission is accompanied by the emission of antineutrino (β- decay) or neutrino (β+ decay), which shares energy and momentum of the decay. The beta emission has a characteristic spectrum. This characteristic spectrum is caused by the fact that either a neutrino or an antineutrino is emitted with emission of beta particle. The shape of this energy curve depends on what fraction of the reaction energy (Q value-the amount of energy released by the reaction) is carried by the massive particle. Beta particles can therefore be emitted with any kinetic energy ranging from 0 to Q. By 1934, Enrico Fermi had developed a Fermi theory of beta decay, which predicted the shape of this energy curve.
Nature of Interaction of Beta Radiation with Matter
Summary of types of interactions:
- Inelastic collisions with atomic electrons (Excitation and Ionization)
- Elastic scattering off nuclei
- Bremsstrahlung.
- Cherenkov radiation.
- Annihilation (only positrons)

Nature of an interaction of a beta radiation with matter is different from the alpha radiation, despite the fact that beta particles are also charged particles. In comparison with alpha particles, beta particles have much lower mass and they reach mostly relativistic energies. Their mass is equal to the mass of the orbital electrons with which they are interacting and unlike the alpha particle a much larger fraction of its kinetic energy can be lost in a single interaction. Since the beta particles mostly reach relativistic energies, the nonrelativistic Bethe formula cannot be used. For high energy electrons an similar expression has also been derived by Bethe to describe the specific energy loss due to excitation and ionization (the “collisional losses”).
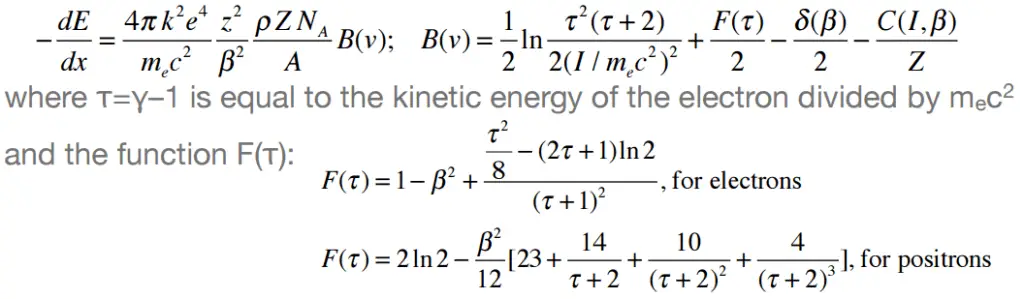
Moreover, beta particles can interact via electron-nuclear interaction (elastic scattering off nuclei), which can significantly change the direction of beta particle. Therefore their path is not so straightforward. The beta particles follow a very zig-zag path through absorbing material, this resulting path of particle is longer than the linear penetration (range) into the material.
Beta particles also differ from other heavy charged particles in the fraction of energy lost by radiative process known as the bremsstrahlung. From classical theory, when a charged particle is accelerated or decelerated, it must radiate energy and the deceleration radiation is known as the bremsstrahlung (“braking radiation”).
There is another mechanism by which beta particles loss energy via production of electromagnetic radiation. When the beta particle moves faster than the speed of light (phase velocity) in the material it generates a shock wave of electromagnetic radiation known as the Cherenkov radiation.
Positrons interact similarly with matter when they are energetic. But when the positron comes to rest, it interacts with a negatively charged electron, resulting in the annihilation of the electron-positron pair.
Bremsstrahlung
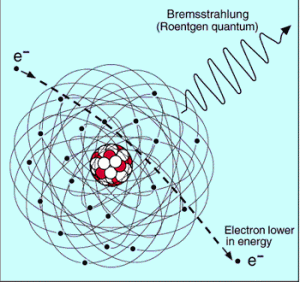
The bremsstrahlung is electromagnetic radiation produced by the acceleration or deceleration of a charged particle when deflected by magnetic fields (an electron by magnetic field of particle accelerator) or another charged particle (an electron by an atomic nucleus). The name bremsstrahlung comes from the German. The literal translation is ‘braking radiation’. From classical theory, when a charged particle is accelerated or decelerated, it must radiate energy.
The bremsstrahlung is one of possible interactions of light charged particles with matter (especially with high atomic numbers).
The two commonest occurrences of bremsstrahlung are by:
- Deceleration of charged particle. When charged particles enter a material they are decelerated by the electric field of the atomic nuclei and atomic electrons.
- Acceleration of charged particle. When ultra-relativistic charged particles move through magnetic fields they are forced to move along a curved path. Since their direction of motion is continually changing, they are also accelerating and so emit bremsstrahlung, in this case it is referred to as synchrotron radiation.
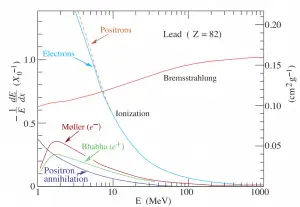
function of electron or positron energy. Source: http://pdg.lbl.gov/
Since the bremsstrahlung is much stronger for lighter particles, this effect is much more important for beta particles than for protons, alpha particles, and heavy charged nuclei (fission fragments). This effect can be neglected at particle energies below about 1 MeV, because the energy loss due to bremsstrahlung is very small. Radiation loss starts to become important only at particle energies well above the minimum ionization energy. At relativistic energies the ratio of loss rate by bremsstrahlung to loss rate by ionization is approximately proportional to the product of the particle’s kinetic energy and the atomic number of the absorber.
The cross section of bremsstrahlung depends on mostly these terms:
So the ratio of stopping powers of bremsstrahlung and ionization losses is:
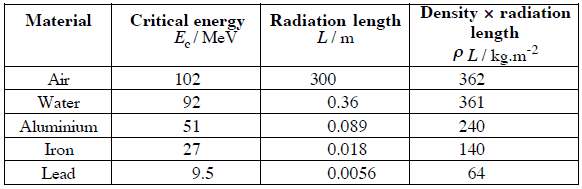
,where E is the particle’s (electron’s) kinetic energy, Z is the mean atomic number of the material and E’ is a proportionality constant; E’ ≈ 800 MeV. The kinetic energy at which energy loss by bremsstrahlung is equal to the energy loss by ionization and excitation (collisional losses) is called the critical energy. Another paremeter is the radiation length, defined as the distance over which the incident electron’s energy is reduced by a factor 1/e (0.37) due to radiation losses alone. Following table give some typical values:
Cherenkov Radiation
The cherenkov radiation is electromagnetic radiation emitted when a charged particle (such as an electron) moves through a dielectric medium faster than the phase velocity of light in that medium. It is similar to the bow wave produced by a boat travelling faster than the speed of water waves. Cherenkov radiation occurs only if the particle’s speed is higher than the phase velocity of light in the material. Even at high energies the energy lost by Cherenkov radiation is much less than that by the other mechanisms (collisions, bremsstrahlung). It is named after Soviet physicist Pavel Alekseyevich Cherenkov, who shared the Nobel Prize in physics in 1958 with Ilya Frank and Igor Tamm for the discovery of Cherenkov radiation, made in 1934.

Cherenkov radiation can be used to detect high-energy charged particles (especially beta particles). In nuclear reactors or in a spent nuclear fuel pool, beta particles (high-energy electrons) are released as the fission fragments decay. The glow is visible also after the chain reaction stops (in the reactor). The cherenkov radiation can characterize the remaining radioactivity of spent nuclear fuel, therefore it can be used for measuring of fuel burnup.
Positron Interactions
 The coulomb forces that constitute the major mechanism of energy loss for electrons are present for either positive or negative charge on the particle and constitute the major mechanism of energy loss also for positrons. Whatever the interaction involves a repulsive or attractive force between the incident particle and orbital electron (or atomic nucleus), the impulse and energy transfer for particles of equal mass are about the same. Therefore positrons interact similarly with matter when they are energetic. The track of positrons in material is similar to the track of electrons. Even their specific energy loss and range are about the same for equal initial energies.
The coulomb forces that constitute the major mechanism of energy loss for electrons are present for either positive or negative charge on the particle and constitute the major mechanism of energy loss also for positrons. Whatever the interaction involves a repulsive or attractive force between the incident particle and orbital electron (or atomic nucleus), the impulse and energy transfer for particles of equal mass are about the same. Therefore positrons interact similarly with matter when they are energetic. The track of positrons in material is similar to the track of electrons. Even their specific energy loss and range are about the same for equal initial energies.
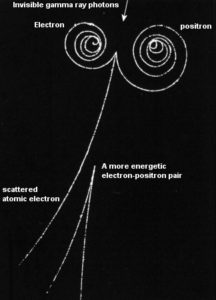
At the end of their path, positrons differ significantly from electrons. When a positron (antimatter particle) comes to rest, it interacts with an electron (matter particle), resulting in the annihilation of the both particles and the complete conversion of their rest mass to pure energy (according to the E=mc2 formula) in the form of two oppositely directed 0.511 MeV gamma rays (photons).
Positron Annihilation
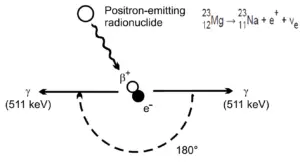
Electron–positron annihilation occurs when a negatively charged electron and a positively charged positron collide.When a low-energy electron annihilates a low-energy positron (antiparticle of electron), they can only produce two or more photons (gamma rays). The production of only one photon is forbidden because of conservation of linear momentum and total energy. The production of another particle is also forbidden because of both particles (electron-positron) together do not carry enough mass-energy to produce heavier particles. When an electron and a positron collide, they annihilate resulting in the complete conversion of their rest mass to pure energy (according to the E=mc2 formula) in the form of two oppositely directed 0.511 MeV gamma rays (photons).
e− + e+ → γ + γ (2x 0.511 MeV)
This process must satisfy a number of conservation laws, including:
- Conservation of electric charge. The net charge before and after is zero.
- Conservation of linear momentum and total energy. T
- Conservation of angular momentum.
We hope, this article, Interaction of Beta Radiation with Matter, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.