Corrosion is the deterioration of a material due to chemical interaction with its environment. It is natural process in which metals convert its structure into a more chemically-stable form such as oxides, hydroxides, or sulfides. The consequences of corrosion are all too common. Familiar examples include the rusting of automotive body panels and pipings and many tools. Corrosion is usually a negative phenomenon, since it is associated with mechanical failure of an object. Metal atoms are removed from a structural element until it fails, or oxides build up inside a pipe until it is plugged. All metals and alloys are subject to corrosion. Even the noble metals, such as gold, are subject to corrosive attack in some environments.
Intergranular Corrosion – Weld Decay
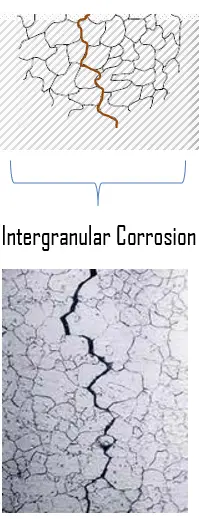 Intergranular corrosion (IGC) is preferential corrosion along the grain boundaries of a material. for some alloys and in specific environments. This type of corrosion is especially prevalent in some stainless steels. In stainless steels, intergranular corrosion may occur as a consequence of the precipitation of chromium carbides (Cr23C6) or intermetallic phases.
Intergranular corrosion (IGC) is preferential corrosion along the grain boundaries of a material. for some alloys and in specific environments. This type of corrosion is especially prevalent in some stainless steels. In stainless steels, intergranular corrosion may occur as a consequence of the precipitation of chromium carbides (Cr23C6) or intermetallic phases.
The resistance of these metallic alloys to the chemical effects of corrosive agents is based on passivation. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 10.5% by weight, above which passivity can occur and below which it is impossible. But the chromium carbides may precipitate in the grain boundaries, which result in depletion of chromium in the zones close to the grain boundaries due to the diffusion rate of chromium that is slow. The chromium-depleted zones become less corrosion resistant than the rest of the matrix. In a corrosive environment the depleted areas may be activated and corrosion will occur in very narrow areas between the grains.
Intergranular corrosion is an especially severe problem in the welding of stainless steels, when it is often termed weld decay. Also a stainless steel, which has been heat-treated in a way that produces grain boundary precipitates and adjacent chromium depleted zones, it is sensitised. Stainless steels can be stabilized against this behavior by addition of titanium, niobium, or tantalum, which form titanium carbide, niobium carbide and tantalum carbide preferentially to chromium carbide, by lowering the content of carbon in the steel and in case of welding also in the filler metal under 0.02%, or by heating the entire part above 1000 °C and quenching it in water, leading to dissolution of the chromium carbide in the grains and then preventing its precipitation.
There are two special cases of intergranular corrosion, but these mechanisms are treated separately:
- Stress corrosion cracking. Intergranular corrosion induced by environmental stresses is termed stress corrosion cracking.
- Chloride stress corrosion cracking. Intergranular corrosion induced by combined action of environmental stresses and chlorine is termed chloride stress corrosion cracking.
Stress Corrosion Cracking – SCC
One of the most serious metallurgical problems and one that is a major concern in the nuclear industry is stress-corrosion cracking (SCC). Stress-corrosion cracking results from the combined action of an applied tensile stress and a corrosive environment, both influences are necessary. SCC is a type of intergranular attack corrosion that occurs at the grain boundaries under tensile stress. It tends to propagate as stress opens cracks that are subject to corrosion, which are then corroded further, weakening the metal by further cracking. The cracks can follow intergranular or transgranular paths, and there is often a tendency for crack branching. Failure behavior is characteristic of that for a brittle material, even though the metal alloy is intrinsically ductile. SCC can lead to unexpected sudden failure of normally ductile metal alloys subjected to a tensile stress, especially at elevated temperature. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when exposed to a small number of chemical environments.
See also: Stress Corrosion Cracking
The most effective means of preventing SCC in reactor systems are:
- designing properly
- reducing stress
- removing critical environmental species such as hydroxides, chlorides, and oxygen
- avoiding stagnant areas and crevices in heat exchangers where chloride and hydroxide might become concentrated.
Chloride Stress Corrosion Cracking
Chloride stress corrosion occurs in austenitic stainless steels under tensile stress in the presence of oxygen, chloride ions, and high temperature. It is one of the most important forms of stress corrosion that concerns the nuclear industry. Austenitic stainless steels contain between 16 and 25% Cr and can also contain nitrogen in solution, both of which contribute to their relatively high uniform corrosion resistance. One type of corrosion which can attack austenitic stainless steel is chloride stress corrosion.
The three conditions that must be present for chloride stress corrosion to occur are as follows:
- Chloride ions are present in the environment
- Dissolved oxygen is present in the environment
- Metal is under tensile stress
Chloride stress corrosion involves selective attack of the metal along grain boundaries. The resistance of these metallic alloys to the chemical effects of corrosive agents is based on passivation. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 10.5% by weight, above which passivity can occur and below which it is impossible. But the chromium carbides may precipitate in the grain boundaries, which result in depletion of chromium in the zones close to the grain boundaries due to the diffusion rate of chromium that is slow. The chromium-depleted zones become less corrosion resistant than the rest of the matrix. In a corrosive environment the depleted areas may be activated and corrosion will occur in very narrow areas between the grains.
It has been found that this is closely associated with certain heat treatments resulting from welding. This can be minimized considerably by proper annealing processes. This form of corrosion is controlled by maintaining low chloride ion and oxygen content in the environment and use of low carbon steels. Ferritic stainless steels are chosen for their resistance to stress corrosion cracking, which makes them an attractive alternative to austenitic stainless steels in applications where chloride-induced SCC is prevalent.
We hope, this article, Intergranular Corrosion – Weld Decay, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.