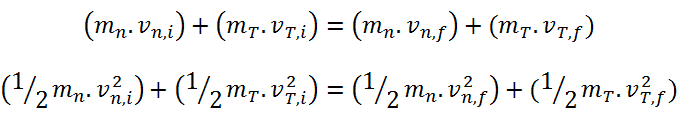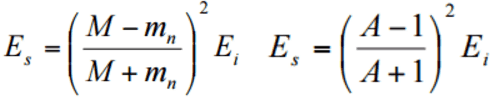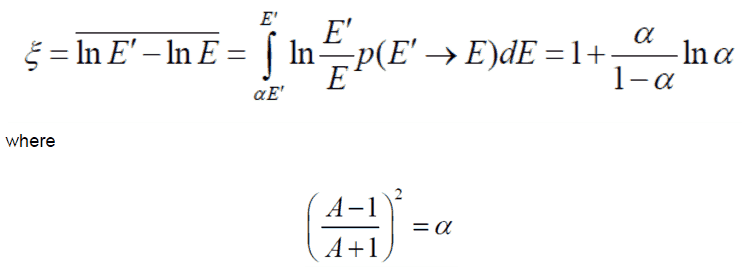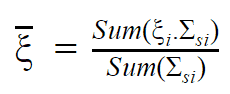Neutron Elastic Scattering
Generally, a neutron scattering reaction occurs when a target nucleus emits a single neutron after a neutron-nucleus interaction. In an elastic scattering reaction between a neutron and a target nucleus, there is no energy transferred into nuclear excitation. The elastic scattering conserves both momentum and kinetic energy of the “system”. It may be modeled as a billiard ball collision between a neutron and a nucleus.
- Potential scattering. In potential scattering, the neutron and the nucleus interact without neutron absorption and the formation of a compound nucleus. In fact, the incident neutron does not necessarily have to “touch” the nucleus and the neutron is scattered by the short range nuclear forces when it approaches close enough to the nucleus. Potential scattering occurs with incident neutrons that have an energy of up to about 1 MeV. It may be modeled as a billiard ball collision between a neutron and a nucleus.
- Compound-elastic scattering. In some cases, if the kinetic energy of an incident neutron just right to form a resonance, the neutron may be absorbed and a compound nucleus may be formed. This interaction is more unusual and is also known as resonance elastic scattering. Due to formation of the compound nucleus, initial and final neutron are not the same.
Key Characteristics of Elastic Scattering
- Elastic scattering is the most important process for slowing down neutrons.
- Total kinetic energy of the system is conserved in elastic scattering.
- In this process, energy lost by the neutron is transferred to the recoiling nucleus.
- Maximum energy transfer is occurred with an head-on collision.
- Kinetic energy of the recoiled nucleus depends on the recoiled angle φ of the nucleus.
- Elastic scattering cross-sections for light elements are more or less independent of neutron energy up to 1 MeV.
- For intermediate and heavy elements, the elastic cross-section is constant at low energy with some specifics at higher energy.
- A good approximation is, σs = const, for all elements, that are of importance.
- At low energy, σs can be described by the one-level Breit-Wigner formula.
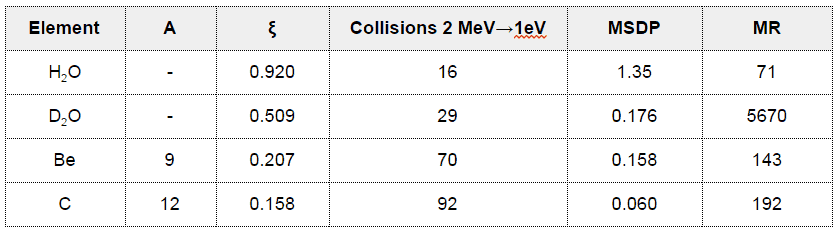
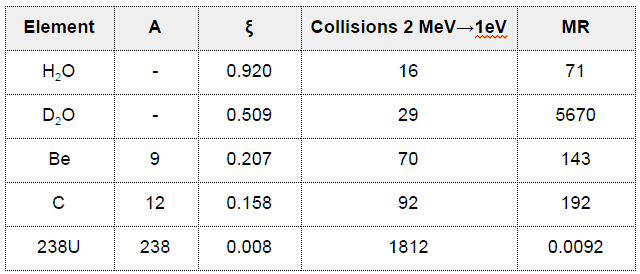
- Nearly all elements have scattering cross-sections in the range of 2 to 20 barns.
- The important exception is for water and heavy water.
- If the kinetic energy of an incident neutron is large compared with the chemical binding energy of the atoms in a molecule, the chemical bound can be ignored.
- If the kinetic energy of an incident neutron is of the order or less than the chemical binding energy, the cross-section of the molecule is not equal to the sum of cross-sections of its individual nuclei.
- Scattering of slow neutrons by molecules is greater than by free nuclei.
- Therefore one nucleus microscopic cross-sections do not describe the process correctly, while the macroscopic cross-section (Σs) has a precise meaning.
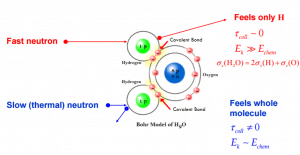 Scattering of slow neutrons by molecules is greater than by free nuclei.
Scattering of slow neutrons by molecules is greater than by free nuclei.
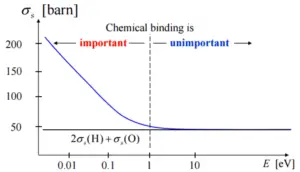
If the kinetic energy of an incident neutron is of the order or less than the chemical binding energy, the cross-section of the molecule is not equal to the sum of cross-sections of its individual nuclei.
Elastic Scattering Cross-section
To be an effective moderator, the probability of elastic reaction between neutron and the nucleus must be high. In terms of cross-sections, the elastic scattering cross section of a moderator’s nucleus must be high.
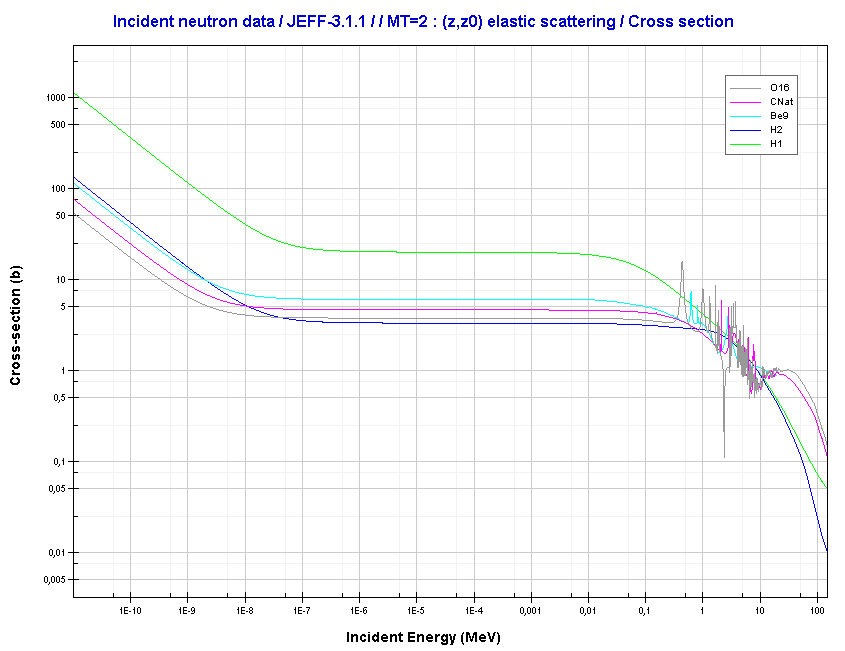 Elastic scattering cross-sections for light elements are more or less independent of neutron energy up to 1 MeV.
Elastic scattering cross-sections for light elements are more or less independent of neutron energy up to 1 MeV.
Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
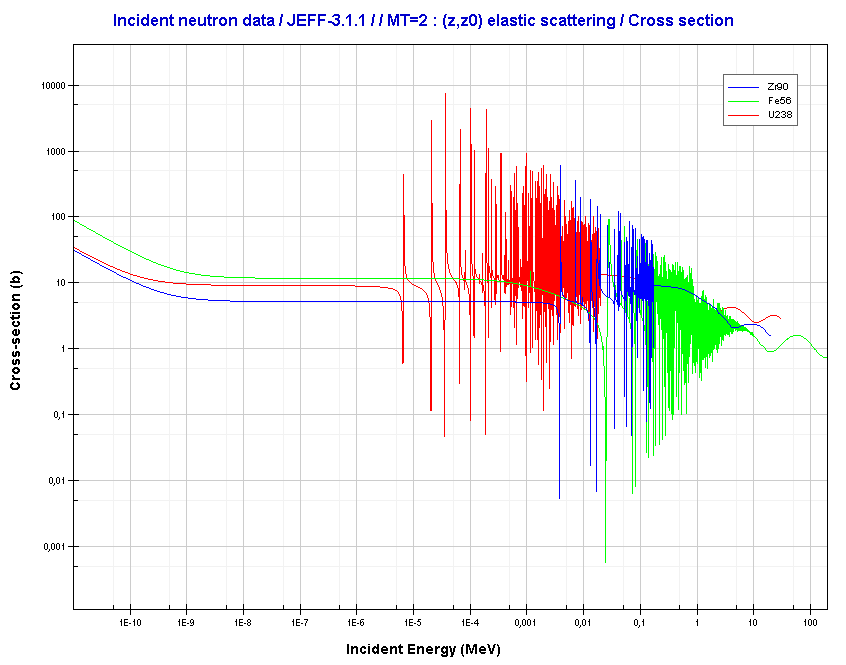 For intermediate and heavy elements, the elastic cross-section is constant at low energy with some specifics at higher energy.
For intermediate and heavy elements, the elastic cross-section is constant at low energy with some specifics at higher energy.
Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
Elastic Scattering and Neutron Moderators
As can be seen, a high elastic scattering cross-section is important, but does not describe comprehensively capabilities of moderators. In order to describe capabilities of a material to slow down neutrons, three new material variables must be defined:
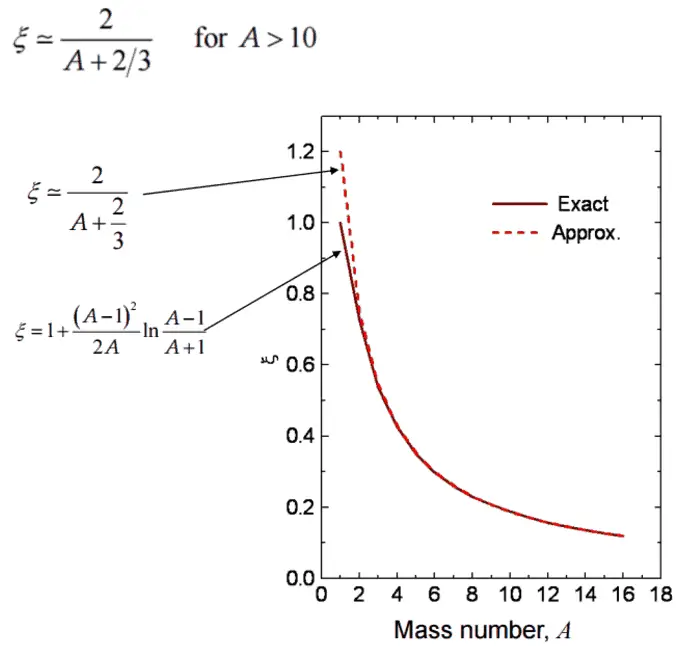
- The Average Logarithmic Energy Decrement (ξ)
- The Macroscopic Slowing Down Power (MSDP)
- The Moderating Ratio (MR)
- high cross-section for neutron scattering
- high energy loss per collision
- low cross-section for absorption
- high melting and boiling point
- high thermal conductivity
- high specific heat capacity
- low viscosity
- low activity
- low corrosive
- cheap
We hope, this article, Neutron Elastic Scattering, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.