 There are metals, that exhibit a passivity to corrosion. Passivity is the characteristic of a metal exhibited when that metal does not become active in the corrosion reaction. Passivation is natural process of the buildup of a stable, tenacious layer of metal oxide or protective barrier on the surface of the metal that acts as a barrier separating the metal surface from the environment. Passivity decreases or stops the corrosion process because of the formation of the layer. Fortunately, from an engineering standpoint, the metals most susceptible to this kind of behavior are the common engineering and structural materials, including iron, nickel, silicon, chromium, titanium, and alloys containing these metals.
There are metals, that exhibit a passivity to corrosion. Passivity is the characteristic of a metal exhibited when that metal does not become active in the corrosion reaction. Passivation is natural process of the buildup of a stable, tenacious layer of metal oxide or protective barrier on the surface of the metal that acts as a barrier separating the metal surface from the environment. Passivity decreases or stops the corrosion process because of the formation of the layer. Fortunately, from an engineering standpoint, the metals most susceptible to this kind of behavior are the common engineering and structural materials, including iron, nickel, silicon, chromium, titanium, and alloys containing these metals.
For example, stainless steel owes its corrosion-resistant properties to the formation of a self-healing passive surface film. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 10.5% by weight, above which passivity can occur and below which it is impossible. Once the surface is cleaned and the bulk composition of the stainless steel is exposed to air, the passive film forms immediately.
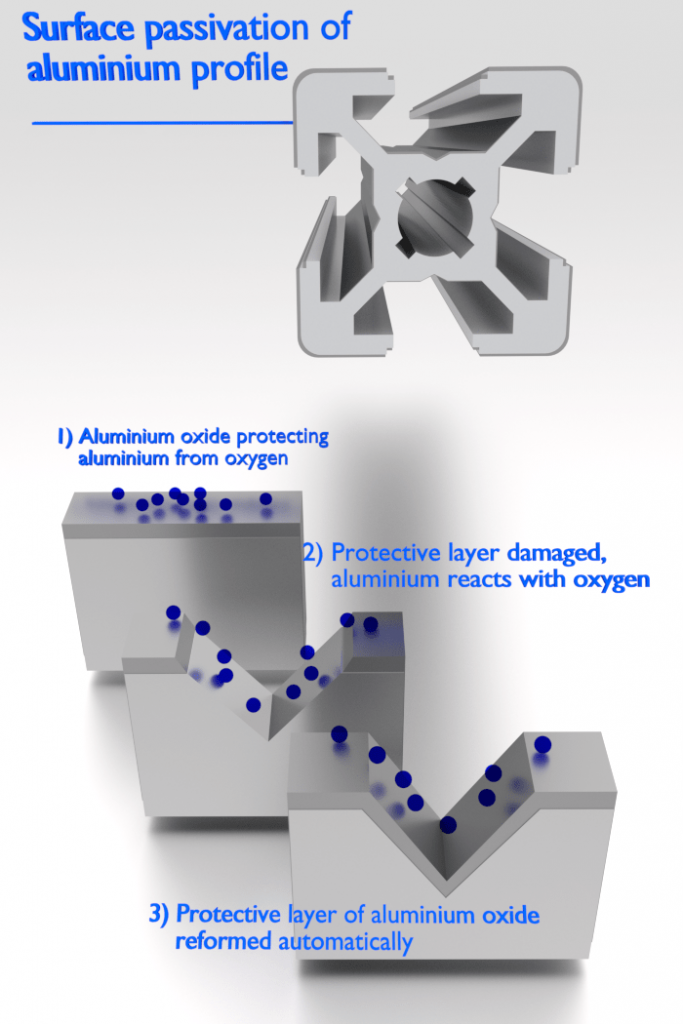
Aluminium is highly corrosion resistant in many environments because it also passivates. If damaged, the protective film normally re-forms very rapidly. However, a change in the character of the environment (e.g., alteration in the concentration of the active corrosive species) may cause a passivated material to revert to an active state. Generally, at high temperatures (in water, corrosion limits the use of aluminium to temperatures near 100°C), the relative low strength and poor corrosion properties of aluminium make it unsuitable as a structural material.
We hope, this article, Passivation of Metals, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.