Potassium is a naturally-occurring chemical element with atomic number 19 which means there are 19 protons and 19 electrons in the atomic structure. The chemical symbol for potassium is K (from Neo-Latin kalium).
Natural potassium consists primarily of isotope K-39 (93.26%), therefore the atomic mass of potassium element is close to the atomic mass of K-39 isotope (39.098 u). Natural potassium also consists of two other isotopes: K-41 (6.73%) and K-40 (0.012%). Potassium-40 is an unstable (radioactive) naturally-occurring isotope of potassium. It has a very long half-life of 1.251×109 years. Therefore, this isotope belong to primordial nuclides, because its half-life is comparable to the age of the Earth.
Traces of K-40 are found in all potassium, and it is the most common radioisotope in the human body. K-40 is a radioactive isotope of potassium which has a very long half-life of 1.251×109 years and undergoes both types of beta decay. From this point of view, also a human body can be considered as a source of antimatter.
- About 89.28% of the time (10.72% is by electron capture), it decays to calcium-40 with emission of a beta particle (β−, an electron) with a maximum energy of 1.33 MeV and an antineutrino, which is an antiparticle to the neutrino.
- Very rarely (0.001% of the time) it will decay to Ar-40 by emitting a positron (β+) and a neutrino.
Potassium-40 inside Body – Radiation Dose
The potassium concentration in the human body is strictly based on the homeostatic principle. Potassium is more or less distributed in the body (especially in soft tissues) following intake in foods. A 70-kg man contains about 126 g of potassium (0.18%), most of that is located in muscles. The daily consumption of potassium is approximately 2.5 gram. Hence the concentration of potassium-40 is nearly stable in all persons at a level of about 55 Bq/kg (3850 Bq in total), which corresponds to the annual effective dose of 0.2 mSv.
Banana Equivalent Dose – BED
Banana equivalent dose, BED, is an informal dose quantity of ionizing radiation exposure. Banana equivalent dose is intended as a general educational example to compare a dose of radioactivity to the dose one is exposed to by eating one average-sized banana. One BED is often correlated to 10-7 Sievert (0.1 µSv).
Bananas contain significantly high potassium concentrations, which is vital for the functioning of all living cells. The transfer of potassium ions through nerve cell membranes is necessary for normal nerve transmission. But natural potassium also contains a radioactive isotope potassium-40 (0.012%). Potassium-40 is a radioactive isotope of potassium which has a very long half-life of 1.251×109 years and undergoes both types of beta decay.
One BED is often correlated to 10-7 Sievert (0.1 µSv). The radiation exposure from consuming a banana is approximately 1% of the average daily exposure to radiation, which is 100 banana equivalent doses (BED). A chest CT scan delivers 58,000 BED (5.8 mSv). A lethal dose, the dose that kills a human with a 50% risk within 30 days (LD50/30) of radiation, is approximately 50,000,000 BED (5000 mSv). However, in practice, this dose is not cumulative, as the principal radioactive component is excreted to maintain metabolic equilibrium. Moreover, there is also a problem with the collective dose.
The BED is only meant to inform the public about the existence of very low levels of natural radioactivity within a natural food and is not a formally adopted dose measurement.
Internal Radiation – Is it dangerous?
We must emphasize, eating bananas, working as airline flight crew or living in locations with, increases your annual dose rate. But it does not mean, that it must be dangerous. In each case, intensity of radiation also matters. It is very similar as for heat from a fire (less energetic radiation). If you are too close, the intensity of heat radiation is high and you can get burned. If you are at the right distance, you can withstand there without any problems and moreover it is comfortable. If you are too far from heat source, the insufficiency of heat can also hurt you. This analogy, in a certain sense, can be applied to radiation also from radiation sources.
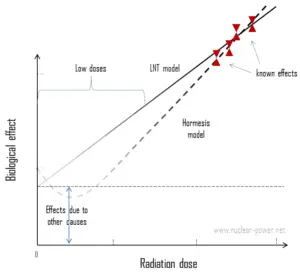
In case of internal radiation, we are talking usually about so called “low doses”. Low dose here means additional small doses comparable to the normal background radiation (10 µSv = average daily dose received from natural background). The doses are very very low and therefore the probability of cancer induction could be almost negligible. Secondly, and this is crucial, the truth about low-dose radiation health effects still needs to be found. It is not exactly known, whether these low doses of radiation are detrimental or beneficial (and where is the threshold). Government and regulatory bodies assume a LNT model instead of a threshold or hormesis not because it is the more scientifically convincing, but because it is the more conservative estimate. Problem of this model is that it neglects a number of defence biological processes that may be crucial at low doses. The research during the last two decades is very interesting and show that small doses of radiation given at a low dose rate stimulate the defense mechanisms. Therefore the LNT model is not universally accepted with some proposing an adaptive dose–response relationship where low doses are protective and high doses are detrimental. Many studies have contradicted the LNT model and many of these have shown adaptive response to low dose radiation resulting in reduced mutations and cancers. This phenomenon is known as radiation hormesis.
We hope, this article, Potassium-40 – Characteristics – Half-life, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.