Protons and Neutrons in a Nucleus
 A nuclear stability is determined by the competition between two fundamental interactions. Protons and neutrons are attracted each other via residual strong force. On the other hand protons repel each other via the electric force due to their positive charge. Therefore neutrons within the nucleus act somewhat like nuclear glue, neutrons attract each other and protons , which helps offset the electrical repulsion between protons. There are only certain combinations of neutrons and protons, which forms stable nuclei. For example, the most common nuclide of the common chemical element lead (Pb) has 82 protons and 126 neutrons.
A nuclear stability is determined by the competition between two fundamental interactions. Protons and neutrons are attracted each other via residual strong force. On the other hand protons repel each other via the electric force due to their positive charge. Therefore neutrons within the nucleus act somewhat like nuclear glue, neutrons attract each other and protons , which helps offset the electrical repulsion between protons. There are only certain combinations of neutrons and protons, which forms stable nuclei. For example, the most common nuclide of the common chemical element lead (Pb) has 82 protons and 126 neutrons.
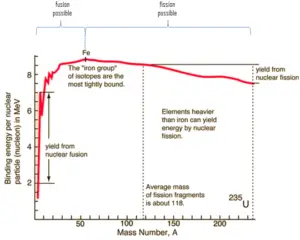
Because of the strength of the nuclear force at short distances, the nuclear binding energy (the energy required to disassemble a nucleus of an atom into its component parts) of nucleons is more than seven orders of magnitude larger than the electromagnetic energy binding electrons in atoms. Nuclear reactions (such as nuclear fission or nuclear fusion) therefore have an energy density that is more than 10 000 000x that of chemical reactions.
Residual Strong Force
The residual strong force, also known as the nuclear force, is a very short range (about 1 to 3 fm) force, which acts to hold neutrons and protons together in nuclei. In nuclei, this force acts against the enormous repulsive electromagnetic force of the protons. It acts equally only between pairs of neutrons, pairs of protons, or a neutron and a proton. The term residual is associated with the fact, it is the residuum of the fundamental strong interaction between the quarks that make up the protons and neutrons. The residual strong force acts indirectly through the virtual π and ρ mesons, which transmit the force between nucleons that holds the nucleus together.
Atomic nuclei consist of protons and neutrons, which attract each other through the nuclear force, while protons repel each other via the electromagnetic force due to their positive charge. These two forces compete, leading to various stability of nuclei. There are only certain combinations of neutrons and protons, which forms stable nuclei. Neutrons stabilize the nucleus, because they attract each other and protons , which helps offset the electrical repulsion between protons. As a result, as the number of protons increases, an increasing ratio of neutrons to protons is needed to form a stable nucleus. If there are too many (neutrons also obey the Pauli exclusion principle) or too few neutrons for a given number of protons, the resulting nucleus is not stable and it undergoes radioactive decay. Unstable isotopes decay through various radioactive decay pathways, most commonly alpha decay, beta decay, or electron capture. Many other rare types of decay, such as spontaneous fission or neutron emission are known.
See also: Liquid Drop Model
We hope, this article, Proton and Neutron in Nucleus, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.