Temperature-entropy Diagrams – T-s Diagrams
In general, the phases of a substance and the relationships between its properties are most commonly shown on property diagrams. A large number of different properties have been defined, and there are some dependencies between properties.
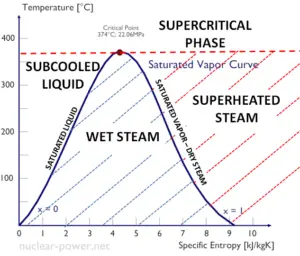
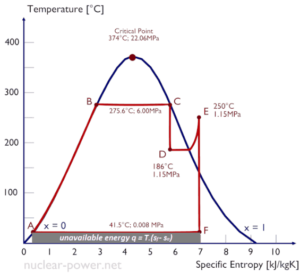
A Temperature-entropy diagram (T-s diagram) is the type of diagram most frequently used to analyze energy transfer system cycles. It is used in thermodynamics to visualize changes to temperature and specific entropy during a thermodynamic process or cycle.
This is because the work done by or on the system and the heat added to or removed from the system can be visualized on the T-s diagram. By the definition of entropy, the heat transferred to or from a system equals the area under the T-s curve of the process.
dQ = TdS
An isentropic process is depicted as a vertical line on a T-s diagram, whereas an isothermal process is a horizontal line. In an idealized state, compression is a pump, compression in a compressor and expansion in a turbine are isentropic processes. Therefore it is very useful in power engineering, because these devices are used in thermodynamic cycles of power plants.
Note that, the isentropic assumptions are only applicable with ideal cycles. Real thermodynamic cycles have inherent energy losses due to inefficiency of compressors and turbines.
We hope, this article, Temperature-entropy Diagram – T-s Diagrams, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.