Terrestrial radiation refers to sources of radiation that are in the soil, water, and vegetation. The major isotopes of concern for terrestrial radiation are potassium, uranium and the decay products of uranium, such as thorium, radium, and radon. Note that, terrestrial radiation includes an external exposure caused by these radionuclides. An internal dose caused by these redionuclides is discussed in: Internal Source of Radiation.
These radionuclides are in trace amounts all around us. When the Earth was formed, a number of radioactive elements were formed. After the four billion years, all the shorter-lived isotopes have decayed. But some of these isotopes have very long half-lives, billions of years, and are still present. These radionuclides are known as primordial radionuclides and contributes to the annual dose to an individual. Because most of the natural radioactive isotopes are heavy, more than one disintegration is necessary before a stable atom is reached. This sequence of unstable atomic nuclei and their modes of decays, which leads to a stable nucleus, is known as the radioactive series.
All, natural radionuclides are usually divided into two groups depending upon their origin:
- Primordial radionuclides. Primordial radionuclides are radionuclides found on the Earth that have existed in their current form since before Earth was formed. Primordial radionuclides are residues from the Big Bang, from cosmogenic sources, and from ancient supernova explosions which occurred before the formation of the solar system. Bismuth, thorium, uranium and plutonium are primordial radionuclides because they have half-lives long enough to still be found on the Earth. Potassium-40 also belongs to primordial nuclides.
- Cosmogenic radionuclides. Cosmogenic radionuclides are those which are continually being produced by the interaction of cosmic rays.
Dose from Terrestrial Radiation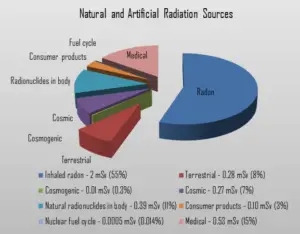
Low levels of uranium, thorium, and their decay products are found everywhere. Some of these materials are ingested with food and water, while others, such as radon, are inhaled. The dose from terrestrial sources also varies in different parts of the world. Locations with higher concentrations of uranium and thorium in their soil have higher dose levels. The average dose rate that originates from terrestrial nuclides (except radon exposure) is about 0.057 µGy/hr. The maximum values have been measured on monazite sand in Guarapari, Brazil (up to 50 µGy/hr and in Kerala, India (about 2 µGy/hr), and on rocks with a high radium concentration in Ramsar, Iran (from 1 to 10 µGy/hr).
The major isotopes of concern for terrestrial radiation are uranium and the decay products of uranium, such as thorium, radium, and radon. Radon is usually the largest natural source of radiation contributing to the exposure of members of the public, sometimes accounting for half the total exposure from all sources. It is so important, that is is usually treated separately. The average annual radiation dose to a person from radon and its decay products is about 2 mSv/year and it may vary over many orders of magnitude from place to place.
Radon – Health Effects
Radon is a colorless, odorless, tasteless noble gas, occurring naturally as the decay product of radium. All isotopes of radon are radioactive, but the two radon isotopes radon-222 and radon-220 are very important from radiation protection point of view.
- Radon-222. The radon-222 isotope is a natural decay product of the most stable uranium isotope (uranium-238), thus it is a member of uranium series.
- Radon-220. The radon-220 isotope, commonly referred to as thoron, is a natural decay product of the most stable thorium isotope (thorium-232), thus it is a member of thorium series.
It is important to note that radon is a noble gas, whereas all its decay products are metals. The main mechanism for the entry of radon into the atmosphere is diffusion through the soil. As a gas, radon diffuses through rocks and the soil. When radon disintegrates, the daughter metallic isotopes are ions that will be attached to other molecules like water and to aerosol particles in the air. Therefore all discussions of radon concentrations in the environment refer to radon-222. While the average rate of production of radon-220 (thoron) is about the same as that of radon-222, the amount of radon-220 in the environment is much less than that of radon-222 because of significantly shorter half-life (it has less time to diffuse) of radon-222 (55 seconds, versus 3.8 days respectively). Simply radon-220 has lower chance to escape from bedrock.
See also: Radon – Health Effects
Radon-222
Radon-222 is a gas produced by the decay of radium-226. Both are a part of the natural uranium series. Since uranium is found in soil throughout the world in varying concentrations, also dose from gaseous radon is varying throughout the world. Radon-222 is the most important and most stable isotope of radon. It has a half-life of only 3.8 days, making radon one of the rarest elements since it decays away so quickly. An important source of natural radiation is radon gas, which seeps continuously from bedrock but can, because of its high density, accumulate in poorly ventilated houses. The fact radon is gas plays a crucial role in spreading of all its daughter nuclei. Simply radon is a transport medium from bedrock to atmosphere (or inside buildings) for its short-lived decay products (Pb-210 and Po-210), that posses much more health risks.
Radioactive Series in Nature
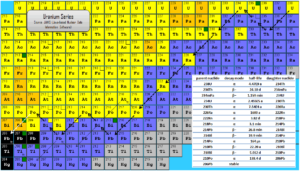 Radioactive series (known also as a radioactive cascades) are three naturally occurring radioactive decay chains and one artificial radioactive decay chain of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. Most radioisotopes do not decay directlyto a stable state and all isotopes within the series decay in the same way. In physics of nuclear decays, the disintegrating nucleus is usually referred to as the parent nucleus and the nucleus remaining after the event as the daughter nucleus. Since alpha decay represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom (which contains four nucleons), there are only four decay series. Within each series, therefore, the mass number of the members may be expressed as four times an appropriate integer (n) plus the constant for that series. As a result, the thorium series is known as the 4n series, the neptunium series as the 4n + 1 series, the uranium series as the 4n + 2 series and the actinium series as the 4n + 3 series.
Radioactive series (known also as a radioactive cascades) are three naturally occurring radioactive decay chains and one artificial radioactive decay chain of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. Most radioisotopes do not decay directlyto a stable state and all isotopes within the series decay in the same way. In physics of nuclear decays, the disintegrating nucleus is usually referred to as the parent nucleus and the nucleus remaining after the event as the daughter nucleus. Since alpha decay represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom (which contains four nucleons), there are only four decay series. Within each series, therefore, the mass number of the members may be expressed as four times an appropriate integer (n) plus the constant for that series. As a result, the thorium series is known as the 4n series, the neptunium series as the 4n + 1 series, the uranium series as the 4n + 2 series and the actinium series as the 4n + 3 series.
Three of the sets are called natural or classical series. The fourth set, the neptunium series, is headed by neptunium-237. Its members are produced artificially by nuclear reactions and do not occur naturally.
- the thorium series (4n series),
- the uranium series (4n+2 series),
- the actinium series (4n+3 series),
- the neptunium series (4n+1 series).
The classical series are headed by primordial unstable nuclei. Primordial nuclides are nuclides found on the Earth that have existed in their current form since before Earth was formed. The previous four series consist of the radioisotopes, that are the descendants of four heavy nuclei with long and very long half-lives:
- the thorium series with thorium-232 (with a half-life of 14.0 billion years),
- the uranium series with uranium-238 (which lives for 4.47 billion years),
- the actinium series with uranium-235 (with a half-life of 0.7 billion years).
- the neptunium series with neptunium-237 (with a half-life of 2 million years).
The half-lives of all the daughter nuclei are all extremely variable, and it is difficult to represent a range of timescales going from individual seconds to billions of years. Since daughter radioisotopes have different half-lives then secular equilibrium is reached after some time. In the long decay chain for a naturally radioactive element, such as uranium-238, where all of the elements in the chain are in secular equilibrium, each of the descendants has built up to an equilibrium amount and all decay at the rate set by the original parent. If and when equilibrium is achieved, each successive daughter isotope is present in direct proportion to its half-life. Since its activity is inversely proportional to its half-life, each nuclide in the decay chain finally contributes as many individual transformations as the head of the chain.
As can be seen from figures, branching occurs in all four of the radioactive series. That means the decay of a given species may occur in more than one way. For example, in the thorium series, bismuth-212 decays partially by negative beta emission to polonium-212 and partially by alpha emission to thallium-206.
Radioactive cascade significantly influences radioactivity (disintegrations per second) of natural samples and natural materials. All the descendants are present, at least transiently, in any natural sample, whether metal, compound, or mineral. For example, pure uranium-238 is weakly radioactive (proportional to its long half-life), but a uranium ore is about 13 times more radioactive than the pure uranium-238 metal because of its daughter isotopes (e.g. radon, radium etc.) it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain they also generate radon, a heavy, inert, naturally occurring radioactive gas. Radon itself is a radioactive noble gas, but the main issue is that it is a transport medium from bedrock to atmosphere (or inside buildings) for its short-lived decay products (Pb-210 and Po-210), that posses much more health risks.
Radiation from Uranium and its Decay Products
 Uranium cascade significantly influences radioactivity (disintegrations per second) of natural samples and natural materials. All the descendants are present, at least transiently, in any natural uranium-containing sample, whether metal, compound, or mineral. For example, pure uranium-238 is weakly radioactive (proportional to its long half-life), but a uranium ore is about 13 times more radioactive than the pure uranium-238 metal because of its daughter isotopes (e.g. radon, radium etc.) it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain they also generate radon, a heavy, inert, naturally occurring radioactive gas.
Uranium cascade significantly influences radioactivity (disintegrations per second) of natural samples and natural materials. All the descendants are present, at least transiently, in any natural uranium-containing sample, whether metal, compound, or mineral. For example, pure uranium-238 is weakly radioactive (proportional to its long half-life), but a uranium ore is about 13 times more radioactive than the pure uranium-238 metal because of its daughter isotopes (e.g. radon, radium etc.) it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain they also generate radon, a heavy, inert, naturally occurring radioactive gas.
Radiation from Thorium and its Decay Products
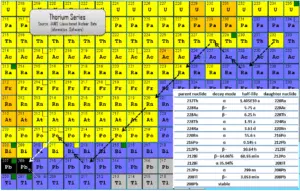 Thorium cascade significantly influences radioactivity(disintegrations per second) of natural samples and natural materials. All the descendants are present, at least transiently, in any natural thorium-containing sample, whether metal, compound, or mineral. For example, pure thorium-232 is weakly radioactive (proportional to its long half-life), but a thorium ore is about 10 times more radioactive than the pure thorium-232 metal because of its daughter isotopes (e.g. radon, radium etc.) it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain they also generate radon, a heavy, inert, naturally occurring radioactive gas.
Thorium cascade significantly influences radioactivity(disintegrations per second) of natural samples and natural materials. All the descendants are present, at least transiently, in any natural thorium-containing sample, whether metal, compound, or mineral. For example, pure thorium-232 is weakly radioactive (proportional to its long half-life), but a thorium ore is about 10 times more radioactive than the pure thorium-232 metal because of its daughter isotopes (e.g. radon, radium etc.) it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain they also generate radon, a heavy, inert, naturally occurring radioactive gas.
Liquid Earth’s Core
 All three naturally-occurring isotopes of uranium (238U, 235U and 234U) and naturally-occurring isotope of thorium have very long half-life (e.g. 4.47×109 years for 238U). Because of this very long half-life uranium and thorium are weakly radioactive and contributes to low levels of natural background radiation in the environment. These isotopes are alpha radioactive (emitting alpha particle), but they can also rarely undergo a spontaneous fission.
All three naturally-occurring isotopes of uranium (238U, 235U and 234U) and naturally-occurring isotope of thorium have very long half-life (e.g. 4.47×109 years for 238U). Because of this very long half-life uranium and thorium are weakly radioactive and contributes to low levels of natural background radiation in the environment. These isotopes are alpha radioactive (emitting alpha particle), but they can also rarely undergo a spontaneous fission.
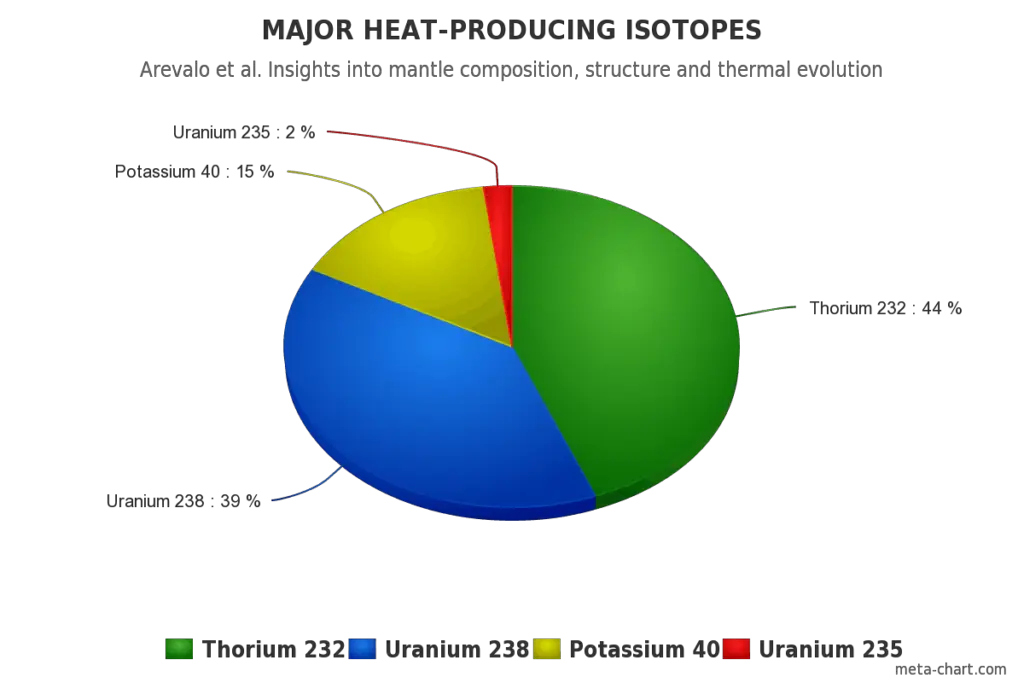
All naturally-occurring isotopes belong to primordial nuclides, because their half-life is comparable to the age of the Earth (~4.54×109 years). Uranium has the second highest atomic mass of these primordial nuclides, lighter only than plutonium. Moreover the decay heat of uranium and thorium and their decay products (e.g. radon, radium etc.) contributes to heating of Earth’s core. Together with potassium-40 in the Earth’s mantle is thought that these elements are the main source of heat that keeps the Earth’s core liquid.
Terrestrial Radiation – Is it dangerous?
We must emphasize, eating bananas, working as airline flight crew or living in locations with, increases your annual dose rate. But it does not mean, that it must be dangerous. In each case, intensity of radiation also matters. It is very similar as for heat from a fire (less energetic radiation). If you are too close, the intensity of heat radiation is high and you can get burned. If you are at the right distance, you can withstand there without any problems and moreover it is comfortable. If you are too far from heat source, the insufficiency of heat can also hurt you. This analogy, in a certain sense, can be applied to radiation also from radiation sources.
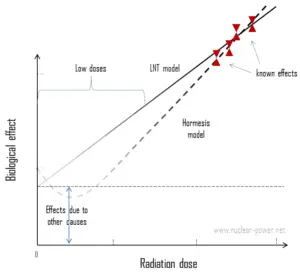
In case of terrestrial radiation, we are talking usually about so called “low doses”. Low dose here means additional small doses comparable to the normal background radiation (10 µSv = average daily dose received from natural background). The doses are very very low and therefore the probability of cancer induction could be almost negligible. Secondly, and this is crucial, the truth about low-dose radiation health effects still needs to be found. It is not exactly known, whether these low doses of radiation are detrimental or beneficial (and where is the threshold). Government and regulatory bodies assume a LNT model instead of a threshold or hormesis not because it is the more scientifically convincing, but because it is the more conservative estimate. Problem of this model is that it neglects a number of defence biological processes that may be crucial at low doses. The research during the last two decades is very interesting and show that small doses of radiation given at a low dose rate stimulate the defense mechanisms. Therefore the LNT model is not universally accepted with some proposing an adaptive dose–response relationship where low doses are protective and high doses are detrimental. Many studies have contradicted the LNT model and many of these have shown adaptive response to low dose radiation resulting in reduced mutations and cancers. This phenomenon is known as radiation hormesis.
We hope, this article, Terrestrial Radiation, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.