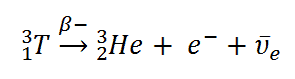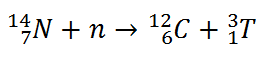Tritium
Tritium is the only naturally-occurring radioisotope of hydrogen. Its atomic number is naturally 1 which means there is 1 proton and 1 electron in the atomic structure. Unlike the hydrogen nucleus and deuterium nucleus, tritium has 2 neutrons in the nucleus. Tritium is naturally-occurring, but it is extremely rare. Tritium is produced in the atmosphere when cosmic rays collide with air molecules. Tritium is also a byproduct of the production of electricity by nuclear power plants. The name of this isotope is formed from the Greek word τρίτος (trítos) meaning “third”.
Decay of Tritium
Tritium is a radioactive isotope, bur it emits a very weak form of radiation, a low-energy beta particle that is similar to an electron. It is a pure beta emitter (i.e. beta emitter without an accompanying gamma radiation). The electron’s kinetic energy varies, with an average of 5.7 keV, while the remaining energy is carried off by the nearly undetectable electron antineutrino. Such a very low energy of electron causes, that the electron cannot penetrate the skin or even does not travel very far in air. Beta particles from tritium can penetrate only about 6.0 mm of air.
Tritium decays via negative beta decay into helium-3 with half-life of 12.3 years.
3H
Tritium in nuclear reactors
Tritium is a byproduct in nuclear reactors. Most important source (due to releases of tritiated water) of tritium in nuclear power plants stems from the boric acid, which is commonly used as a chemical shim to compensate an excess of initial reactivity. Main reactions, in which the tritium is generated from boron are below:
10B(n,T + 2*alpha)
This threshold reaction of fast neutron with an isotope 10B is the main way, how radioactive tritium in primary circuit of all PWRs is generated. 10B is the principal source of radioactive tritium in primary circuit of all PWRs (which use boric acid as a chemical shim). 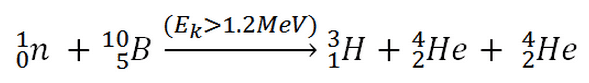 Note that, this reaction occurs very rarely in comparison with the most common (n,alpha) reaction of isotope 10B with thermal neutrons.
Note that, this reaction occurs very rarely in comparison with the most common (n,alpha) reaction of isotope 10B with thermal neutrons.
There are more reactions with neutrons, which can rarely lead to formation of radioactive tritium, for example:
10B(n,alpha)7Li + 7Li(n,n+alpha)3H – threshold reaction (~3 MeV).
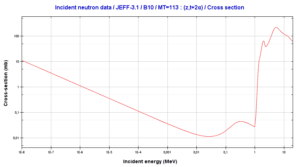
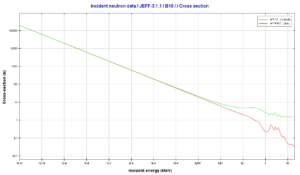 Boron 10. Comparison of total cross-section and cross-section for (n,alpha) reactions.
Boron 10. Comparison of total cross-section and cross-section for (n,alpha) reactions.Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library[/captionBoron 10. Comparison of total cross-section and cross-section for (n,alpha) reactions.
Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
Tritium is also a fission product (ternary fission) of the splitting of fissionable materials. In fact, fission probably produces more tritium than all other sources in Light Water Reactors. Its production (yield) is of about one atom per each 10,000 fissions. On the other hand only a very small fraction of the fission-product tritium diffuses out of the fuel matrix and fuel cladding into the primary coolant.Tritium is also produced in reaction with 6Li.
6Li(n,α)3H
This is a reaction allowing detection of neutrons, but in some cases, LiOH is added to control the pH of primary coolant in some LWR. The reaction cross-section for thermal neutrons is σ = 925 barns and the natural lithium has abundance of 6Li 7,4%.
Tritium occurs in nuclear power plants in the form of tritiated water. Tritiated water is like normal water, but is very very weakly radioactive. Therefore it dose not pose a hazard to human health. The releases of tritiated water are closely monitored by plant operators and state supervisors.
Reference: Jacobs D.G. Sources of Tritium and Its Behaviour Upon Release to the Environment. US Atomic Energy Commission, 1968.
Tritium in Nature
Tritium is produced in the atmosphere when cosmic rays collide with air molecules. In the most important reaction for natural production, a fast neutron (which must have energy greater than 4.0 MeV) interacts with atmospheric nitrogen:
Worldwide, the production of tritium from natural sources is 148 petabecquerels per year. In result, the tritiated water produced participates in the water cycle.
- about 400 Bq/m3 in continental water
- about 100 Bq/m3 in oceans
Tritium poses a risk to health as a result of internal exposure only following ingestion in drinking water or food, or inhalation or absorption through the skin. The tritium taken into the body is uniformly distributed among all soft tissues. An average annual dose from natural tritium intake is 0.01 μSv.
In case of artificial tritium ingestion or inhalation, a biological half-time of tritium is 10 days for HTO and 40 days for OBT (organically bound tritium) formed from HTO in the body of adults. It was also shown that the biological half-time of HTO depends strongly on many variables and varies from about 4 to 18 days. During the warmer months, the average half-life is lower, which is attributed to increased water intake. As well as, drinking larger amounts of alcohol will reduce the biological half-life of water in the body.
See also: Tritium in Nature
We hope, this article, Tritium, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.