Uranium 238
Uranium 238, which alone constitutes 99.28% of natural uranium is the most common isotope of uranium in the nature. This isotope has the longest half-life (4.47×109 years) and therefore its abundance is so high. 238U belongs to primordial nuclides, because its half-life is comparable to the age of the Earth (~4.5×109 years). For its very long half-life, it is still present in the Earth’s crust.238U decays via alpha decay (by way of thorium 234 and protactinium 234) into 234U. 238U occasionally decays by spontaneous fission with probability of 0.000055%.
238U is a fissionable isotope, but is not fissile isotope. 238U is not capable of undergoing fission reaction after absorbing thermal neutron, on the other hand 238U can be fissioned by fast neutron with energy higher than >1MeV. 238U does not meet also alternative requirement to fissile materials. 238U is not capable of sustaining a nuclear fission chain reaction, because too many of neutrons produced by fission of 238U have lower energies than original neutron.
238U belongs also to the group of fertile isotopes. Radiative capture of a neutron leads to the formation of fissile 239Pu. This is the way how 238U contributes to the operation of nuclear reactors and production of electricity through this plutonium. For example, at a burnup of 40GWd/tU, about 40% of the total energy released comes from bred plutonium. This corresponds to a breeding ratio for this fuel burnup of about 0.4 to 0.5. This effect extends the cycle length for such fuels to sometimes nearly twice what it would be otherwise.
See also: Comparison of cross-sections
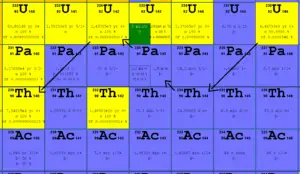 Uranium 238 decays via alpha decay (by way of thorium-234 and protactinium-234) into 234U.
Uranium 238 decays via alpha decay (by way of thorium-234 and protactinium-234) into 234U.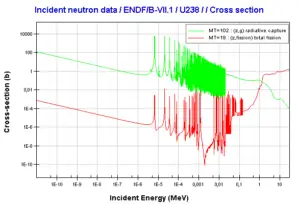 Uranium 238. Comparison of total fission cross-section and cross-section for radiative capture.
Uranium 238. Comparison of total fission cross-section and cross-section for radiative capture.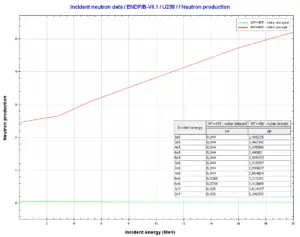 Neutron production per one fast fission of uranium 238.
Neutron production per one fast fission of uranium 238.We hope, this article, Uranium 238, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about materials and their properties.
