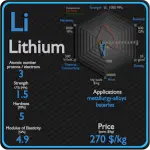
This article contains comparison of key thermal and atomic properties of phosphorus and magnesium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Phosphorus vs Magnesium.
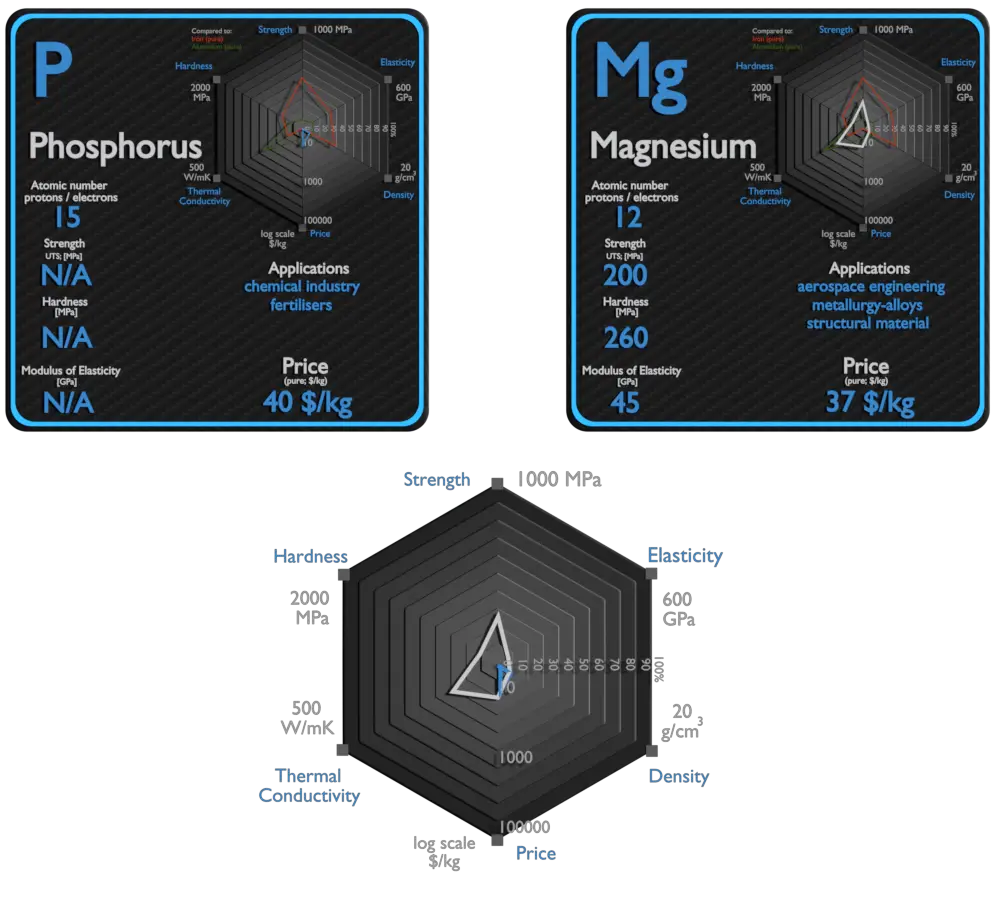
Phosphorus and Magnesium – About Elements

Source: www.luciteria.com
Phosphorus and Magnesium – Applications
Phosphorus
Phosphorus is an essential plant nutrient (the most often limiting nutrient, after nitrogen), and the bulk of all phosphorus production is in concentrated phosphoric acids for agriculture fertilisers, containing as much as 70% to 75% P2O5. The vast majority of phosphorus compounds mined are consumed as fertilisers. Phosphate is needed to replace the phosphorus that plants remove from the soil, and its annual demand is rising nearly twice as fast as the growth of the human population. Other applications include organophosphorus compounds in detergents, pesticides, and nerve agents.
Magnesium
Magnesium is the third-most-commonly-used structural metal, following iron and aluminium.[35] The main applications of magnesium are, in order: aluminium alloys, die-casting (alloyed with zinc), removing sulfur in the production of iron and steel, and the production of titanium in the Kroll process. Magnesium alloys are used in a wide variety of structural and nonstructural applications. Structural applications include automotive, industrial, materials-handling, commercial, and aerospace equipment. Magnesium alloys are used for parts that operate at high speeds and thus must be light weight to minimize inertial forces. Commercial applications include hand-held tools, laptops, luggage, and ladders, automobiles (e.g., steering wheels and columns, seat frames, transmission cases). Magnox (alloy), whose name is an abbreviation for “magnesium non-oxidizing”, is 99% magnesium and 1% aluminum, and is used in the cladding of fuel rods in magnox nuclear power reactors.
Phosphorus and Magnesium – Comparison in Table
| Element | Phosphorus | Magnesium |
| Density | 1.823 g/cm3 | 1.738 g/cm3 |
| Ultimate Tensile Strength | N/A | 200 MPa |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | N/A | 45 GPa |
| Mohs Scale | 0.5 | 2.5 |
| Brinell Hardness | N/A | 260 MPa |
| Vickers Hardness | N/A | N/A |
| Melting Point | 44.1 °C | 649 °C |
| Boiling Point | 280 °C | 1090 °C |
| Thermal Conductivity | 0.235 W/mK | 156 W/mK |
| Thermal Expansion Coefficient | N/A | 24.8 µm/mK |
| Specific Heat | 0.77 J/g K | 1.02 J/g K |
| Heat of Fusion | 0.657 kJ/mol | 8.954 kJ/mol |
| Heat of Vaporization | 51.9 kJ/mol | 127.4 kJ/mol |